
2H-Pyran-4(3H)-one synthesis
- Product Name:2H-Pyran-4(3H)-one
- CAS Number:84302-42-1
- Molecular formula:C5H6O2
- Molecular Weight:98.1
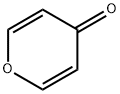
108-97-4
75 suppliers
$61.00/250mg

84302-42-1
16 suppliers
inquiry
Yield: 33%
Reaction Conditions:
with hydrogen;5% palladium/carbon hydrated in ethanol;toluene at 20; for 1 h;
Steps:
7 Example 7
Example 7 (Synthesis of dihydropyran-4-one) In a flask made of glass having an inner volume of 20 ml and equipped with a stirring device, a thermometer, a reflux condenser and a balloon filled with hydrogen were charged 3.0 g (31.2 mmol) of pyran-4-one, 0.6 g (50% hydrated product; containing 0.14 mmol as a palladium atom) of 5% by weight palladium/carbon, 30 ml of toluene and 3 ml of ethanol, and the mixture was reacted under hydrogen atmosphere at room temperature for 1 hour. After completion of the reaction, the reaction mixture was filtered, and the filtrate was concentrated. The concentrate was purified by silica gel column chromatography (Eluent; hexane:ethyl acetate=10:1) to give 1.0 g (Isolation yield; 33%) of dihydropyran-4-one as colorless liquid. Physical properties of dihydropyran-4-one are as follows. CI-MS (m/e); 99 (M+1) 1H-NMR (CDCl3, δ (ppm)); 2.57 to 2.63 (2H, m), 4.50 (2H, dd, J=7.6Hz, 6.8Hz), 5.41 (1H, d, J=6.1Hz), 7.35 (1H, d, J=6.1Hz)
References:
Ube Industries, Ltd. EP1700853, 2006, A1 Location in patent:Page/Page column 13
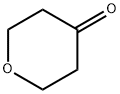
29943-42-8
418 suppliers
$5.00/1g

84302-42-1
16 suppliers
inquiry
![1,5,9-Trioxaspiro[5.5]undec-7-ene, 3,3-dimethyl-](/CAS/20210305/GIF/106569-79-3.gif)
106569-79-3
0 suppliers
inquiry

84302-42-1
16 suppliers
inquiry
![Silane, [(3-ethoxy-1-methylene-2-propenyl)oxy]trimethyl-, (E)- (9CI)](/CAS/20210305/GIF/73431-56-8.gif)

![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)