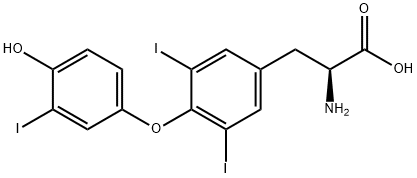
3,3',5-Triiodo-L-thyronine synthesis
- Product Name:3,3',5-Triiodo-L-thyronine
- CAS Number:6893-02-3
- Molecular formula:C15H12I3NO4
- Molecular Weight:650.97
55-06-1
294 suppliers
$10.00/25mg

6893-02-3
251 suppliers
$19.00/250mg
Yield:-
Reaction Conditions:
with acetic acid in water;Large scale;
Steps:
2
All quantities of raw materials are expressed for 1 kg of Levoditi. Iodine (approx.0.48 kg, source: SQM), Nal (approx.0.65 kg, source: Ajay - SQM) and water were charged in a reactor 18-22°C and stirred until complete dissolution. The resulting iodinating mixture was maintained under stirring at room temperature until use. Levoditi obtained from L-thyrosine according to the process described in: Chalmers, J. R. et al. A. J. Chem. Soc. 1949, 3424-3433), Nal (approx. 0.32 kg) and water were charged in another reactor and 70% monoethylamine was added. The iodinating mixture was added to the reaction mixture. The suspension obtained was stirred for at least 6 h at 18-22°C, then was cooled to 0°C over 1 hr, stirred for 3-4 hrs and filtered. The cake was washed with water. The wet solid was suspended in water and acetic acid was added to the mixture and stirred. The suspension was filtered and the cake washed with water. The wet solid was re-suspended in stirred water, filtered and washed with water. The cake was then suspended in DMAC (approx. 12.15 kg) and the suspension was anhydrified by distilling under vacuum. The suspension was cooled to 5-10°C and, under nitrogen atmosphere, CSA (approx. 1.54 kg) was slowly added and the temperature was maintained below 15°C. The solution was heated to 18-22°C for 1 h and maintained for another 1 hour, then was added to a reactor containing a solution of Na2CO3 (approx. 2.27 kg) in water (approx. 29.02 kg), previously prepared, while maintaining the temperature of 30°C. The solution was purified onto a column of Amberlite XAD 1600 (12.5 L) by elution of water (87.5 L) and water/acetone mixture (125 L) while decreasing polarity starting from 95:5 to 70:30. The fractions of high HPLC purity were collected and distilled under vacuum until the desired composition was achieved (approx.0.04 kg T3S /L suspension). The suspension was cooled to 40°C and Ethanol (approx. 5.22 kg) was added, to obtain a solution. The mixture was cooled to 0°C over 2h, & precipitated, stirred for another hour and then filtered. The cake was washed with Ethanol/water mixture at room temperature. Wet solid was dried at approximately 40°C under vacuum. 0.98 kg of pure T3-Sulfate sodium salt (HPLC Area % > 99%) was obtained as a white solid.
References:
JP5833222,2015,B2 Location in patent:Paragraph 0060
300-30-1
61 suppliers
inquiry

6893-02-3
251 suppliers
$19.00/250mg