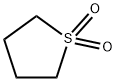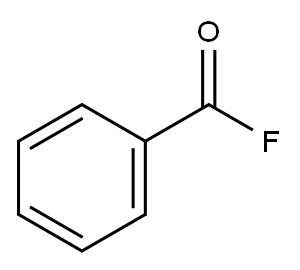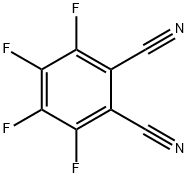
3,4,5,6-Tetrafluorophthalonitrile synthesis
- Product Name:3,4,5,6-Tetrafluorophthalonitrile
- CAS Number:1835-65-0
- Molecular formula:C8F4N2
- Molecular Weight:200.09
Yield:1835-65-0 74%
Reaction Conditions:
with potassium fluoride in water
Steps:
A 3,4,5,6-Tetrafluorophthalonitrile
Example A 3,4,5,6-Tetrafluorophthalonitrile Anhydrous potassium fluoride (11.0 kg) is added to a 50 gallon stainless steel reactor. The salt is dried under 28 inches vacuum at 115°-138° C. for 48 hours. The salt is cooled to 100° C. and tetramethylenesulfone (19 liters) added followed by tetrachlorophthalonitrile (4.74 kg). The mixture is heated with stirring to 156° C. over a 30 minute period. Heating with vigorous agitation is continued for another 2.5 hours at 135°-162° C. The mixture was cooled to 31° C. (15 minutes) and ice (69 kg) and demineralized water (119 liters) were added. The resulting mixture was stirred 1.5 hours before centrifuging to collect crude product which was washed with demineralized water (120 liters). The crude product was transferred back into the 50 gallon stainless steel still and demineralized water (100 liters) added. The mixture was steam distilled until 80 liters of distillate were collected. The distillate was cooled to 0°-5° C. and the product collected on a centrifuge. The crystals were washed with demineralized water (2*90 liters) to give 2.82 kg wet product: LOD 6.4%; calculated yield: 74%. A small sample was dried under vacuum for two days at room temperature; mp 81°-83° C.
References:
Warner-Lambert Company US4782180, 1988, A
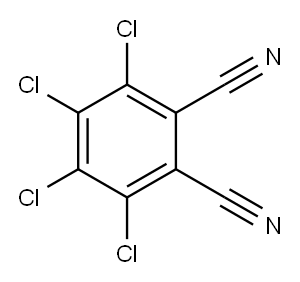
1953-99-7
144 suppliers
$6.00/1g

1835-65-0
247 suppliers
$6.00/1g

827-08-7
153 suppliers
$15.00/250mg
544-92-3
242 suppliers
$9.00/1g

1835-65-0
247 suppliers
$6.00/1g