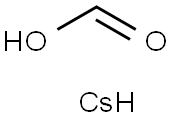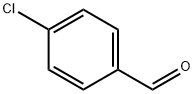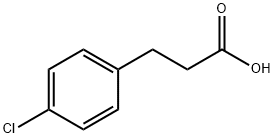
3-(4-Chlorophenyl)propanoic acid synthesis
- Product Name:3-(4-Chlorophenyl)propanoic acid
- CAS Number:2019-34-3
- Molecular formula:C9H9ClO2
- Molecular Weight:184.62

133793-08-5
0 suppliers
inquiry

2019-34-3
216 suppliers
$6.00/1g
Yield:2019-34-3 98%
Reaction Conditions:
with hydrogen in ethyl acetate at 40; for 1.5 h;
Steps:
2
General procedure: 123.3 mg (0.5 mmol) of benzyl 4-chlorobenzoate as a substrate and 10.6 mg of 5% Pd / beta zeolite obtained in Reference Example 1 (1 mol% as metallic palladium based on the substrate) as a reduction catalyst Take it in a test tube, add 1 mL of ethyl acetate and suspend it, then pierce the needle attached with a balloon filled with hydrogen gas into the septum at the top of the test tube and separate the gas in the system from another needle stabbed in the septum The pull-out operation was repeated three times, the interior of the system was replaced with hydrogen gas, and then the inside of the test tube was filled with hydrogen gas.After vigorously stirring at 60 ° C. for 24 hours, the resulting reaction solution was filtered using a membrane filter (manufactured by Millipore, Millex-LH, pore diameter 0.45 μm), and the membrane filter was further washed with ethyl acetate (15 mL). The obtained filtrate was concentrated, and the obtained concentrate was subjected to 1 H-NMR.From the obtained spectrum, when the recovery rate of the raw material and the yield of 4-chlorobenzoic acid (the benzyl ester was hydrocracked) obtained as the product were calculated, the conversion of the raw material was 100% The yield of 4-chlorobenzoic acid was 100% (77.6 mg). In Example 1, instead of benzyl 4-chlorobenzoate, benzyl 4-chloro cinnamate136.4 mg (0.5 mmol) of 5% Pd / beta zeolite, 21.2 mg(2 mol% as metallic palladium relative to the substrate), and the reaction was carried out at 40 ° C. for 1.5 hours, the reaction, the treatment after the reaction and the analysis of the product were carried out in the same manner as in Example 1. As a result, the conversion of the raw material was 100%, and 3- (4-chlorophenyl) propionic acid was obtained in a yield of 98% (89.5 mg).
References:
JP5785045,2015,B2 Location in patent:Paragraph 0033; 0034; 0035; 0036; 0037; 0038

1615-02-7
321 suppliers
$7.00/5g

2019-34-3
216 suppliers
$6.00/1g
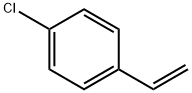
1073-67-2
297 suppliers
$7.00/5g
201230-82-2
1 suppliers
inquiry

2019-34-3
216 suppliers
$6.00/1g

938-95-4
86 suppliers
$31.80/1gm: