
3,4-DIMETHOXYBENZHYDROL synthesis
- Product Name:3,4-DIMETHOXYBENZHYDROL
- CAS Number:56139-08-3
- Molecular formula:C15H16O3
- Molecular Weight:244.29

108-86-1
496 suppliers
$10.00/5g
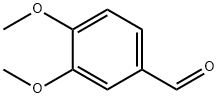
120-14-9
747 suppliers
$5.00/25g

56139-08-3
22 suppliers
$115.00/250mg
Yield:56139-08-3 80%
Reaction Conditions:
Stage #1: bromobenzenewith iodine;magnesium in diethyl ether at 0; for 0.166667 h;
Stage #2: 3,4-dimethoxy-benzaldehyde in diethyl ether; for 12 h;
Steps:
General procedure for the preparation of 1a, 1b, 1c, 1g and 1h:
General procedure: A 50 mL round-bottom flask was charged with bromobenzene (157 mg, 1 mmol), anhydrous Et2O (10 mL), magnesium powder (24 mg, 1 mmol) and a little iodine added. The mixture was cooled to 0 °C and stirred for 10 min, then a solution of 3-methoxybenzaldehyde (136 mg, 1 mmol) and anhydrous Et2O was added slowly. The solution was stirred for 12 h after the temperature rose to room temperature; 5 mL NH4Cl aqueous solution was dropped slowly, and the mixture extracted with Et2O (3×30 mL). The combined organic layers were dried with anhydrous Na2SO4, filtered, and concentrated in vacuo. The residue was isolated by flash column chromatography on silica gel to give the products.
References:
Zhang, Wei;Zhang, Wenxue;Dai, Yisi;Zhu, Haizhen [Tetrahedron Letters,2013,vol. 54,# 13,p. 1747 - 1750] Location in patent:supporting information

120-14-9
747 suppliers
$5.00/25g
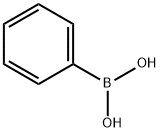
98-80-6
705 suppliers
$5.00/5g

56139-08-3
22 suppliers
$115.00/250mg

120-14-9
747 suppliers
$5.00/25g
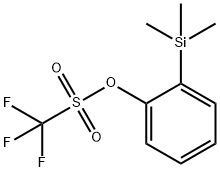
88284-48-4
162 suppliers
$9.00/250mg

56139-08-3
22 suppliers
$115.00/250mg

120-14-9
747 suppliers
$5.00/25g

100-58-3
183 suppliers
$18.00/10ml

56139-08-3
22 suppliers
$115.00/250mg

4038-14-6
186 suppliers
$12.00/5g

56139-08-3
22 suppliers
$115.00/250mg