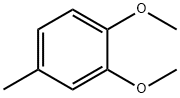
3,4-Dimethoxytoluene synthesis
- Product Name:3,4-Dimethoxytoluene
- CAS Number:494-99-5
- Molecular formula:C9H12O2
- Molecular Weight:152.19
Yield:494-99-5 73.5%
Reaction Conditions:
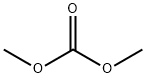
with caesium carbonate in acetonitrile at 160; under 0 - 8550.86 Torr; for 2 h;Microwave irradiation;Reagent/catalyst;Time;
Steps:
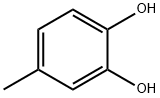
2.3 Example 2: Methylation of benzenedmh with dimethyl-carbonate and CS2CO3 under micro wa ve conditions
Example 2: Methylation of benzenedmh with dimethyl-carbonate and CS2CO3 under micro wa ve conditions Procedure: A solution of substrate (1.82 mmol; Table 2), CS2CG3 ( 1 equiv/OH), DMC (2 mL), and CHjCN' (2 ml), and mesitylene std. ( 100 μΤ) was heated in a 10 mL airtight glass vessel in an Anto Paar Monowave 300 microwave synthesis reactor at 1 0 °C with a stir rate of 600 rpm. The reaction products were analysed by GC-FID and GC-MS. The pressure of the reaction mixture increased from 0-1 1.4 bar over the course of 120 minutes. After 120 minutes, the reaction mixtures were cooled to room temperature, pressure released and the reaction mixture was analysed by GC-FID and GC-MS. Results are shown in Table 2. Table 2 Entrv Substrate % yield Time {mm) 1 catechol 66.5 120 2 hydroquinoae 69.6 120 4-iaeifaylcaiechol 73.5 120 4 methyJhydroquinorte 90.3 1.20 5 resorcinoi 24.1 120 6 2,3-diineiliyihydroquinone 63.7 120 7 4-tthvlcatechoi 78.6 120 The yields of the compounds used in Table 2 are significant and no minor products, e.g., guaiacol, were observed. The numbers correspond to isolated yields.
References:
THE UNIVERSITY OF SYDNEY;MASCHMEYER, Thomas;LOKARE, Kapil, Shyam;MASTERS, Anthony, Frederick WO2015/51402, 2015, A1 Location in patent:Page/Page column 36

93-03-8
373 suppliers
$5.00/1g

494-99-5
185 suppliers
$12.00/25g
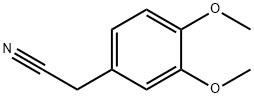
93-17-4
423 suppliers
$7.00/10g

494-99-5
185 suppliers
$12.00/25g
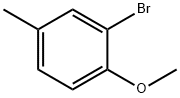
22002-45-5
160 suppliers
$27.00/10g
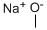
124-41-4
683 suppliers
$12.00/25g

494-99-5
185 suppliers
$12.00/25g