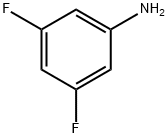
3,5-Difluoroaniline synthesis
- Product Name:3,5-Difluoroaniline
- CAS Number:372-39-4
- Molecular formula:C6H5F2N
- Molecular Weight:129.11
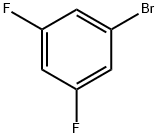
461-96-1
475 suppliers
$6.00/10g

372-39-4
366 suppliers
$16.00/5g
Yield:372-39-4 94%
Reaction Conditions:
with copper(I) oxide;1-D-O-Methyl-chiro-inositol;ammonia;sodium hydroxide in water at 100; for 12 h;
Steps:
1.5. General procedures for ammonization of aryl bromides (Table 4)
General procedure: A 25 mL hydrothermal synthesis reactor equipped with a magnetic stirring bar was charged with sodium hydroxideand 5 mL 25 % ammonia, and then stirred at ambient temperature. After the dissolution of cuprous oxide, L-Que andaryl bromides were added. The reaction mixture was stirred at 100 oC until the hydrolysis of aryl iodides completes byTLC analysis. Then the reaction mixture was cooled to ambient temperature, diluted by water, and extracted with ethylacetate. The combined organic phase was dried over anhydrous magnesium sulfate and the solvent was removed underreduced pressure. The crude products were purified by flash column chromatography on silica gel to afford the desiredproduct.
References:
Bao, Xuefei;Chen, Guoliang;Dong, Jinhua;Du, Fangyu;Li, Hui;Liang, Xinjie;Wu, Ying;Zhang, Yongsheng [Tetrahedron Letters,2020] Location in patent:supporting information

2265-94-3
225 suppliers
$10.00/1g

372-39-4
366 suppliers
$16.00/5g

1977-85-1
2 suppliers
inquiry

372-39-4
366 suppliers
$16.00/5g
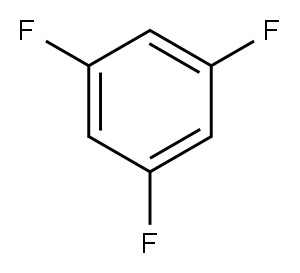
372-38-3
305 suppliers
$9.00/1g

372-39-4
366 suppliers
$16.00/5g

