
3,5-Dimethoxypyridine synthesis
- Product Name:3,5-Dimethoxypyridine
- CAS Number:18677-48-0
- Molecular formula:C7H9NO2
- Molecular Weight:139.15
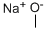
124-41-4
681 suppliers
$12.00/25g
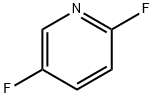
84476-99-3
272 suppliers
$8.00/1g

18677-48-0
123 suppliers
$20.00/100mg
Yield:18677-48-0 91%
Reaction Conditions:
with methanol at 135; for 0.25 h;microwave;
Steps:
To a vial charged with 2,5-difluoropyridine (127 mg, 1.1 mmol) was added a 25% solution of sodium methoxide in methanol (2 mL). Upon completion of addition, the reaction mixture was heated at 135 0C under microwave conditions for 15 min. At the conclusion of this period, the reaction mixture was diluted with brine (5 mL) and extracted with EtOAc (3 x 5 mL). The combined extracts were dried over Na2SO4 and filtered. The volatiles were removed under reduced pressure to provide a residue. The residue was subjected to chromatography on silica gel eluting with 0 to 60% EtOAc/hexanes to provide Intermediate 23 as a yellow oil (139 mg, 91%). 1H NMR (400 MHz, CDCl3) δ ppm 3.80 (s, 6 H), 6.87 (t, J-2.20 Hz, 1 H), 7.78 (d, J=2.20 Hz, 2 H).
References:
BRISTOL-MYERS SQUIBB COMPANY WO2007/30582, 2007, A2 Location in patent:Page/Page column 88
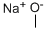
124-41-4
681 suppliers
$12.00/25g

71902-33-5
167 suppliers
$6.00/1g

18677-48-0
123 suppliers
$20.00/100mg
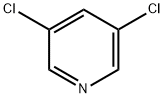
2457-47-8
299 suppliers
$6.00/5g

18677-48-0
123 suppliers
$20.00/100mg

2457-47-8
299 suppliers
$6.00/5g
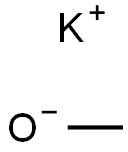
865-33-8
160 suppliers
$12.50/100G

18677-48-0
123 suppliers
$20.00/100mg

2457-47-8
299 suppliers
$6.00/5g
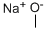
124-41-4
681 suppliers
$12.00/25g

18677-48-0
123 suppliers
$20.00/100mg