3,5-Dinitrobenzonitrile synthesis
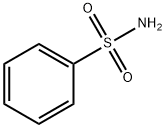
- Product Name:3,5-Dinitrobenzonitrile
- CAS Number:4110-35-4
- Molecular formula:C7H3N3O4
- Molecular Weight:193.12
The same authors also reported that alkyl, benzylic, aryl, and heteroaryl aldoximes bearing various functionalities could be readily converted to the corresponding nitriles using diphosgene in good yields of 82–96%.
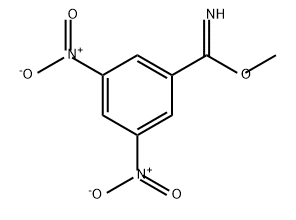
86475-51-6
0 suppliers
inquiry

4110-35-4
130 suppliers
$19.00/1g
Yield:-
Steps:
Multi-step reaction with 3 steps
1: 83 percent / NH4Cl / methanol / 12 h / 55 - 60 °C
2: 7 percent / NaCl, NaOCl / dimethylsulfoxide; hexane; diethyl ether / 1.) 0 deg C, 1.5 h, 2.) room temp, 20 min.
3: NaN3, 2-nitropropane sodium salt / dimethylsulfoxide / 0.01 h / 17 °C
References:
Creary, Xavier [Journal of Organic Chemistry,1993,vol. 58,# 27,p. 7700 - 7708]

121-81-3
144 suppliers
$50.00/500mg

4110-35-4
130 suppliers
$19.00/1g

14193-18-1
56 suppliers
inquiry

4110-35-4
130 suppliers
$19.00/1g