
3-Bromo-5-methoxypyridine synthesis
- Product Name:3-Bromo-5-methoxypyridine
- CAS Number:50720-12-2
- Molecular formula:C6H6BrNO
- Molecular Weight:188.02

625-92-3
477 suppliers
$6.00/10g
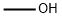
67-56-1
734 suppliers
$7.29/5ml-f

50720-12-2
351 suppliers
$11.00/5g
Yield:50720-12-2 73%
Reaction Conditions:
with sodium hydride in N,N-dimethyl-formamide;mineral oil at 20 - 90; for 1 h;
Steps:
j00643j 3-Bromo-5-methoxypyridine (2):
1006441 A suspension of 60% sodium hydride (11.4 g, 285 mmol) in DMF (450 mL) was charged with methanol (11.5 mL, 285 mmol) at room temperature and heated to 60°C. The resulting solution was charged with 3,5-dibromopyridine (45 g, 190 mmol) and heated to 90°Cfor 1 h. The reaction mixture was cooled to room temperature, diluted with water (50 mL) and extracted with diethyl ether (3 X 50 mL). The combined organic layers were washed with brine, dried over anhydrous Na2504, filtered and concentrated in vacuo resulting in crude compound which was purified by chromatography on silica gel, eluting with 5-10% ethyl acetate in hexane to give 26.1 g, 73% yield of the title compound as an off white solid. ‘H NMR (400 MHz, CDC13): ö = 8.27 (d, J= 17.20 Hz, 2 H), 7.36 (s, 1 H), 3.86 (br. s, 3 H); MS (E5): m/z = 187.85 [M-Hf; LCMS: tR = 1.81 mm.
References:
COFERON, INC.;FOREMAN, Kenneth, W.;JIN, Meizhong;WANNER, Jutta;WERNER, Douglas, S. WO2015/106292, 2015, A1 Location in patent:Paragraph 00634; 00643; 00644

625-92-3
477 suppliers
$6.00/10g

50720-12-2
351 suppliers
$11.00/5g

625-92-3
477 suppliers
$6.00/10g
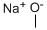
124-41-4
681 suppliers
$12.00/25g

50720-12-2
351 suppliers
$11.00/5g

625-92-3
477 suppliers
$6.00/10g
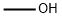
67-56-1
734 suppliers
$7.29/5ml-f
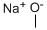
124-41-4
681 suppliers
$12.00/25g

50720-12-2
351 suppliers
$11.00/5g
624-28-2
714 suppliers
$5.00/5g

50720-12-2
351 suppliers
$11.00/5g