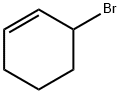
3-BROMOCYCLOHEXENE synthesis
- Product Name:3-BROMOCYCLOHEXENE
- CAS Number:1521-51-3
- Molecular formula:C6H9Br
- Molecular Weight:161.04

110-83-8
289 suppliers
$11.00/25g
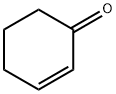
930-68-7
253 suppliers
$9.00/1g
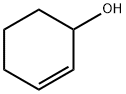
822-67-3
103 suppliers
$49.60/1g

1521-51-3
174 suppliers
$14.00/1g
Yield:-
Reaction Conditions:
with tert.-butylhydroperoxide;Bromotrichloromethane;C68H60Cu4N24O4(4+)*4NO3(1-)*8H2O in water;acetonitrile at 10; under 3800.26 Torr;Reagent/catalyst;Temperature;
Steps:
2.6.1 Oxidation of cyclohexene
General procedure: Compounds 1 and 2 were investigated as catalyst precursors for the oxidation of cyclohexene to 2-cyclohexen-1-ol and 2-cyclohexen-1-one using aqueous tert-butyl hydroperoxide (TBHP) under mild conditions (Scheme 3). Cyclohexene has been studied as a model substrate in view of the importance of the products (i.e., α,β-unsaturated allylic oxidation products used for further additive reactions) [18-19]. The reaction mixtures were prepared as follows: to 0.5-20μmol (preferably 10μmol) of complex 1or 2, contained in a reaction flask, were added 3mL of MeCN, 5.0mmol cyclohexene and 1-10.0mmol TBHP (70% aqueous solution), in this order. The reaction mixture was stirred for 10h at the desired temperature (ca. 30-60°C) under an air (atmospheric pressure), then 50μL of cyclopentanone (as an internal standard) and 9.5mL of diethyl ether (to extract the substrate and the products from the reaction mixture) were added. The resulting mixture was stirred for 15min, and then a sample taken from the organic phase was analyzed by GC. The GC analyses showed the presence of only traces (less than 3%) of oxidation products. The reaction under pressurized dioxygen was carried out in a 13.0mL stainless steel autoclave, equipped with a Teflon-coated magnetic stirring bar. The autoclave was closed and flushed with dioxygen three times to remove the air, and finally pressurized with 5atm of dioxygen. The reaction mixture was stirred for 10h at 50°C using a magnetic stirrer and an oil bath, whereupon it was cooled in an ice bath, degassed, opened and transferred to a flask. Diethy ether (9.5mL) and 50μL of cyclopentanone (GC internal standard) were added. The obtained mixture was vigorously stirred for 10min, and the organic layer was analyzed by gas chromatography (internal standard method). Blank experiments were performed and confirmed that no cyclohexene oxidation products (or only traces, below 0.5%) were obtained in the absence of the metal catalyst.
References:
Jana, Atanu;Konar, Saugata;Das, Kinsuk;Ray, Sangita;Golen, James A.;Rheingold, Arnold L.;El Fallah, Mohamed Salah;Mukherjee, Sanghamitra;Gupta, Samik;Pombeiro, Armando J.L.;Kar, Susanta Kumar [Polyhedron,2013,vol. 62,p. 51 - 60]

110-83-8
289 suppliers
$11.00/25g

1521-51-3
174 suppliers
$14.00/1g

822-67-3
103 suppliers
$49.60/1g

1521-51-3
174 suppliers
$14.00/1g

110-83-8
289 suppliers
$11.00/25g
7429-37-0
63 suppliers
$18.69/5g

1521-51-3
174 suppliers
$14.00/1g

3112-85-4
194 suppliers
$15.00/5g

1521-51-3
174 suppliers
$14.00/1g