
3-Dimethylaminophenylboronic acid synthesis
- Product Name:3-Dimethylaminophenylboronic acid
- CAS Number:178752-79-9
- Molecular formula:C8H12BNO2
- Molecular Weight:165
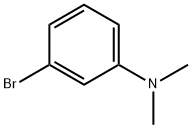
16518-62-0
164 suppliers
$12.00/1g

178752-79-9
144 suppliers
$20.00/1g
Yield:178752-79-9 61%
Reaction Conditions:
Stage #1:3-bromo-N,N-dimethylaniline with n-butyllithium;Trimethyl borate in tetrahydrofuran;hexanes at -78 - -20; for 1.41667 h;
Stage #2: with hydrogenchloride in tetrahydrofuran;hexanes;water for 0.0833333 h;
Steps:
10.a
3-Bromo-N,N-dimethylaniline (500 mg, 2.50 mmol) was dissolved in THF (8 mL) and the solution was cooled to -78° C. n-Butyllithium (1.6M in hexanes, 1.56 mL, 3.75 mmol) was added drop-wise at -78° C. and the reaction mixture was stirred at this temperature for another 5 min. Trimethylborate (1.11 mL, 10.0 mmol) was added and the mixture was stirred for 1 h at -78° C. and was then left to warm to -20° C. over a period of 20 min. 2M HCl (3 mL) was added and the mixture was stirred for 5 min and was then neutralized by the addition of sat. NaHCO3 solution. The mixture was extracted with AcOEt (3×10 mL) and the combined organics were concentrated under reduced pressure to provide the crude boronic acid. Flash chromatography on silica gel eluting with acetone/CH2Cl2 1:1 provided 3-dimethylaminophenylboronic acid (251 mg, 61%).
References:
Kelly, Martha;Lee, Younghee;Liu, Bin;Fujimoto, Ted;Freundlich, Joel;Dorsey, Bruce D.;Flynn, Gary A.;Husain, Arifa US2006/270686, 2006, A1 Location in patent:Page/Page column 26
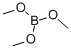
121-43-7
345 suppliers
$13.00/25mL

16518-62-0
164 suppliers
$12.00/1g

178752-79-9
144 suppliers
$20.00/1g