
3-Heptanone synthesis
- Product Name:3-Heptanone
- CAS Number:106-35-4
- Molecular formula:C7H14O
- Molecular Weight:114.19
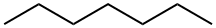
142-82-5
518 suppliers
$16.00/25ML

106-35-4
129 suppliers
$32.00/25mL
Yield:-
Reaction Conditions:
with N-hydroxyphthalimide;oxygen in acetonitrile at 120; under 7500.75 Torr; for 6 h;Autoclave;
Steps:
2.3. Catalytic oxidation of cyclohexane to AA by NHPI-solvent systems
General procedure: Liquid phase catalytic oxidations were carried out in a Teflonlined100 mL stainless steel reactor equipped with a magnetic stirrer.Typically, 3.2 g of cyclohexane, 6.4 g of solvent and 0.6 g ofNHPI catalyst were charged in the autoclave, and then heated tothe reaction temperature (120 C) under magnetic stirring(800 rpm). Oxygen was then charged into the reactor to the desiredpressure (1.0 MPa). The reaction was terminated by switching offthe stirrer and immediately cooling the reactor in an ice-waterbath. The reaction mixture was dissolved in ethanol and filtered.The components of the liquid phase such as unreacted cyclohexaneand KA-oil intermediate were quantitatively analyzed on a Shimadzu2010 plus gas chromatograph with a DB-17 polysiloxanecapillary column (30 m 0.32 mm 0.50 lm) and flame ionizationdetector (FID) using chlorobenzene as the internal standard.The injector and detector temperature were 250 C, and the columntemperature was 100 C. The yield of cyclohexanol was calculatedas a difference between the value obtained by GC and theconcentration of cyclohexyl hydroperoxide determined iodometrically (reduction in cyclohexyl hydroperoxide with PPh3 gives anadditional amount of cyclohexanol). The AA and overoxidationproducts (succinic acid, glutaric acid and valeric acid) were quantitativelyanalyzed according to the external standard methodusing an Agilent 1200 series high-performance liquid chromatographyinstrument equipped with a UV detector (Agilent G1365BMWB) and a column of Zorbax Eclipse XDB C18(150 mm 4.6 mm i.d.). The eluent was 0.01 molL-1 KH2PO4 in a1:9 methanol/water mixture and a flow rate set at 1.0 mL/min,and ultraviolet detector set at 212 nm. To detect possible componentsof oxygen formed, the gases in the reactor were also collectedwith a gas bag after termination of the reactions and thenanalyzed by gas chromatography with a TCD detector. The productswere satisfactorily identified by comparing the MS spectrawith those of the authentic samples. In addition, after the solventwas removed under reduced pressure, the isolation of AA on apreparative scale is carried out as the following procedure. Acetonitrile(20 mL) was added to the reaction mixture to give a whitesolid. After filtration, 0.64 g of AA was obtained.
References:
Liang, Futong;Zhong, Wenzhou;Xiang, Liping;Mao, Liqiu;Xu, Qiong;Kirk, Steven Robert;Yin, Dulin [Journal of Catalysis,2019,vol. 378,p. 256 - 269]
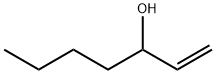
4938-52-7
94 suppliers
$50.00/1 SAMPLE

106-35-4
129 suppliers
$32.00/25mL

123-05-7
125 suppliers
$10.00/5mL

106-35-4
129 suppliers
$32.00/25mL

7642-10-6
17 suppliers
inquiry
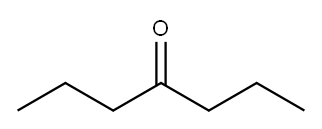
123-19-3
172 suppliers
$16.00/25mL

106-35-4
129 suppliers
$32.00/25mL

123-05-7
125 suppliers
$10.00/5mL

589-82-2
118 suppliers
$50.00/1 SAMPLE
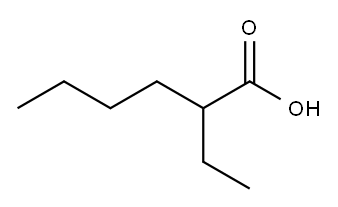
149-57-5
511 suppliers
$5.00/25g

106-35-4
129 suppliers
$32.00/25mL