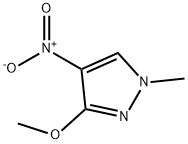
3-methoxy-1-methyl-4-nitropyrazole synthesis
- Product Name:3-methoxy-1-methyl-4-nitropyrazole
- CAS Number:1201935-85-4
- Molecular formula:C5H7N3O3
- Molecular Weight:157.13
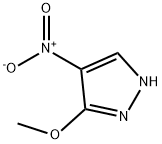
400755-41-1
79 suppliers
$59.00/100mg
74-88-4
339 suppliers
$15.00/10g

1201935-85-4
41 suppliers
$38.00/100mg
Yield: 81%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 25;Inert atmosphere;
Steps:
17.17-2 Example 17-2: 3-Methoxy-1-methyl-4-nitro-1H-pyrazole
Compound 17-1 (10 g, 70 mmol) was dissolved in DMF (50 mL), methyl iodide (4 mL, 77 mmol), potassium carbonate (14.8 g, 105 mmol) were added, under nitrogen atmosphere. The reaction mixture was stirred for 2 hours at 25°C. After the reaction was completed, monitored by TLC, then the reaction mixture was cooled to room temperature. The reaction solution was poured into water (50 mL), extracted with ethyl acetate (50 mL x 2). The combined organic phases were washed with water (100 mL) and saturated brine (100 mL) sequentially, and dried with anhydrous sodium sulfate, then filtered. The filtrate was evaporated under reduced pressure. The crude product was purified by column (petroleum ether/ethyl acetate = 1/1) to give a pale yellow solid 17-2 (8.9 g, yield 81%). 1H NMR (400 MHz, CDCl3) δ 7.92 (s, 1H), 3.96 (s, 3H), 3.73 (s, 3H).
References:
Shanghai Ennovabio Pharmaceuticals Co., Ltd. EP3854793, 2021, A1 Location in patent:Paragraph 0098-0100