
4-(3,4-Dichlorophenyl)-1-tetralone synthesis
- Product Name:4-(3,4-Dichlorophenyl)-1-tetralone
- CAS Number:79560-19-3
- Molecular formula:C16H12Cl2O
- Molecular Weight:291.17

866018-46-4
19 suppliers
inquiry

79560-19-3
274 suppliers
$6.00/5g
Yield:79560-19-3 98%
Reaction Conditions:
with oxygen;sodium hydride in tetrahydrofuran at 0 - 20; for 5 h;
Steps:
4.2. Aerobic oxidation of secondary aryl alcohols
General procedure: A round-bottom flask that was flame-dried and cooled under dry air or oxygen atmosphere was charged with alcohol 6 or 33 (0.5 mmol) and THF (2.5 mL). After stirring at 0 °C for 5 min, NaH powder (1 mmol, 2 equiv) was added in one portion, and the mixture was allowed to warm to room temperature. The reaction was quenched by addition of saturated NH4Cl (2 mL) after the indicated time, extracted with EtOAc (10 mL×2), and washed by brine (15 mL). The combined organic phase was dried over MgSO4, the solvent was removed under vacuum, and the residue was purified by flash chromatography on silica gel to give the desired ketone product 7 and 34. For isolations of acids 8 and 35/36: the combined aqueous phase was acidified with 2 M HCl, and then extracted with EtOAc (10 mL×2) and washed by brine (15 mL). The combined organic phase was dried over Na2SO4, the solvent was removed under vacuum to give acid product that can be further purified by flash chromatography on silica gel.
References:
Wang, Xinbo;Wang, David Zhigang [Tetrahedron,2011,vol. 67,# 19,p. 3406 - 3411] Location in patent:supporting information; experimental part

79560-18-2
31 suppliers
$53.00/100mg

79560-19-3
274 suppliers
$6.00/5g

79560-20-6
108 suppliers
inquiry

871838-58-3
15 suppliers
$185.00/1mg

91797-63-6
3 suppliers
inquiry

79560-19-3
274 suppliers
$6.00/5g

91797-60-3
1 suppliers
inquiry
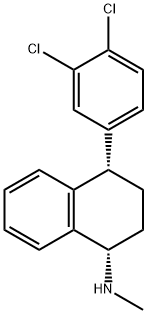
79617-96-2
157 suppliers
inquiry

90-15-3
698 suppliers
$9.00/5g

79560-19-3
274 suppliers
$6.00/5g
