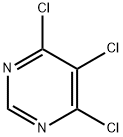
4,5,6-Trichloropyrimidine synthesis
- Product Name:4,5,6-Trichloropyrimidine
- CAS Number:1780-27-4
- Molecular formula:C4HCl3N2
- Molecular Weight:183.42
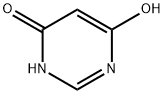
1193-24-4
535 suppliers
$6.00/25g

1780-27-4
147 suppliers
$11.00/100mg
Yield:1780-27-4 86%
Reaction Conditions:
with sulfuryl dichloride;triethylamine;trichlorophosphate in chlorobenzene at 40 - 83; for 19 h;Product distribution / selectivity;
Steps:
2
Example 2; To a 1000 mL four-neck flask, 89.7 g of 4,6-dihydroxypyrimidine and 179.3 g of chlorobenzene were added. The obtained mixture was adjusted at 40°C, and then, 129.6 g of sulfuryl chloride was added dropwise thereto over 1 hour. The obtained mixture was maintained at the same temperature for 6 hours. To the obtained reaction mixture, 269.9 g of phosphorus oxychloride was added at the same temperature. Further, 178.1 g of triethylamine was added dropwise thereto over 2 hours at an inner temperature of 40 to 80°C. After completion of the addition, the obtained mixture was maintained at 83°C for 10 hours. The obtained reaction mixture was cooled to room temperature. To another 100 mL four-neck flask, 269.0 g of water was added followed by adjusting at 40°C. To it, the obtained reaction mixture was added dropwise over 30 minutes. The inner temperature during the addition was 30 to 50°C. The obtained mixture was filtrated using Radiolite (registered trademark) and the obtained filtrate was separated to an organic layer and an aqueous layer. The aqueous layer was extracted with 44.8 g of chlorobenzene, and the obtained chlorobenzene layer was mixed with the previously obtained organic layer. The organic layer after mixing was washed with 44.8 g of water and then, concentrated under reduced pressure to obtain 169.2 g of black oily matter. The oily matter was analyzed by high performance liquid chromatography internal standard method, and 126.9 g of 4,5,6-trichlorpyrimidine was containd in the oily matter. The yield was 86%.
References:
EP2128141,2009,A1 Location in patent:Page/Page column 7
4316-93-2
272 suppliers
$12.00/5g

1780-27-4
147 suppliers
$11.00/100mg

1193-56-2
45 suppliers
$78.00/100mg

1780-27-4
147 suppliers
$11.00/100mg

88982-91-6
53 suppliers
$75.00/250mg

1780-27-4
147 suppliers
$11.00/100mg

63447-41-6
3 suppliers
inquiry

1780-27-4
147 suppliers
$11.00/100mg