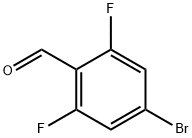
4-Bromo-2,6-difluorobenzaldehyde synthesis
- Product Name:4-Bromo-2,6-difluorobenzaldehyde
- CAS Number:537013-51-7
- Molecular formula:C7H3BrF2O
- Molecular Weight:221
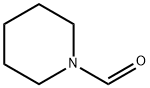
2591-86-8
293 suppliers
$14.00/5g
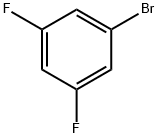
461-96-1
475 suppliers
$6.00/10g

537013-51-7
224 suppliers
$10.00/100mg
Yield:537013-51-7 78%
Reaction Conditions:
Stage #1:3,5-difluorobromobenzene with lithium diisopropyl amide in tetrahydrofuran at -70; for 0.5 h;
Stage #2:N-Formylpiperidine at -70 - 0;
Steps:
1.1 4-bromo-2,6-difluorobenzaldehyde (1)
55 ml (0.11 mmol) of 2 M lithium diisopropylamide are added with stirring at -70° C. to a solution of 19.3 g (0.1 mmol) of 1-bromo-3,5-difluorobenzene in 120 ml of dried tetrahydrofuran.After 30 minutes, N-formylpiperidine is added dropwise at this temperature.The mixture is allowed to warm to 0° C. At about 0° C., the reaction mixture is poured into cold water, acidified using 10% HCl and extracted twice with methyl tert-butyl ether.The combined organic phases are washed with water, dried over Na2SO4 and filtered, and the solvent is removed under reduced pressure.The residue is filtered through SiO2 (heptane/dichloromethane 1:1), (yield: 17.6 g, 78%).
References:
Merck Patent GmbH US2004/6235, 2004, A1 Location in patent:Page 11
162744-59-4
168 suppliers
$8.00/1g

537013-51-7
224 suppliers
$10.00/100mg

461-96-1
475 suppliers
$6.00/10g

537013-51-7
224 suppliers
$10.00/100mg

461-96-1
475 suppliers
$6.00/10g
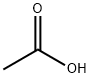
64-19-7
1530 suppliers
$10.00/25ML

537013-51-7
224 suppliers
$10.00/100mg

57848-46-1
381 suppliers
$9.00/1g

537013-51-7
224 suppliers
$10.00/100mg