
4-Bromo-2-chlorobenzonitrile synthesis
- Product Name:4-Bromo-2-chlorobenzonitrile
- CAS Number:154607-01-9
- Molecular formula:C7H3BrClN
- Molecular Weight:216.46
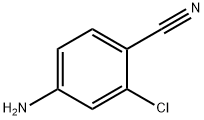
20925-27-3
155 suppliers
$10.00/5g

154607-01-9
265 suppliers
$6.00/1g
Yield:154607-01-9 72%
Reaction Conditions:
Stage #1: 4-amino-2-chlorobenzonitrilewith hydrogenchloride;sodium nitrite in water at 0 - 5;
Stage #2: with hydrogenchloride;copper(I) bromide at 0 - 20; for 2 h;
Steps:
General procedure for the synthesis of 4-Bromo-2-(substituted) benzonitrile (5a, b
General procedure: 4-Cyano-3-(substituted) aniline 4a or 4b (38 mmol) was dissolved in conc. HCl (27 ml). After cooling to 0 C, sodium nitrite (39 mmol, 1.5 eq) was added very slowly to the stirred solution with the temperature being kept within (0 and 5 °C). This diazonium salt solution was then poured into a flask containing CuBr (53 mmol, 1.4 eq) and 22 ml of conc. HCl. The solution was stirred for 2 h, and then, the reaction mixture was poured into ice water (50 ml) extracted with Ethyl acetate (100 ml). The organic layer was separated, and the aqueous layer was extracted with ethyl acetate (2 x 50 ml). The combined extracts were washed with saturated aqueous NaHCO3 solution (1 x 150 ml), brine (3 x 100 ml) and dried over Na2SO4. The resulting material was concentrated, and the residue was purified by silica gel chromatography (petroleum ether:ethyl acetate, 10:1) to afford the title compound (5a or 5b) as white solid (72-75 % yield).
References:
Elancheran;Saravanan;Choudhury, Bhaswati;Divakar;Kabilan;Ramanathan;Das, Babulal;Devi;Kotoky, Jibon [Medicinal Chemistry Research,2016,vol. 25,# 4,p. 539 - 552]
623-00-7
465 suppliers
$6.00/10g

154607-01-9
265 suppliers
$6.00/1g
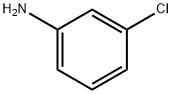
108-42-9
450 suppliers
$10.00/5g

154607-01-9
265 suppliers
$6.00/1g

21402-26-6
236 suppliers
$9.00/1g

154607-01-9
265 suppliers
$6.00/1g

62473-92-1
62 suppliers
$59.00/100mg
874483-75-7
0 suppliers
inquiry

154607-01-9
265 suppliers
$6.00/1g