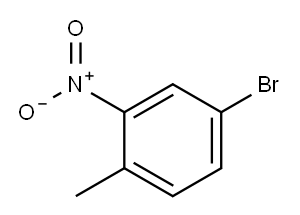
4-Bromo-2-nitrotoluene synthesis
- Product Name:4-Bromo-2-nitrotoluene
- CAS Number:60956-26-5
- Molecular formula:C7H6BrNO2
- Molecular Weight:216.03

119-32-4
253 suppliers
$15.00/25g

60956-26-5
293 suppliers
$8.00/10g
Yield:60956-26-5 89%
Reaction Conditions:
Stage #1:4-Methyl-3-nitroanilin with hydrogen bromide in water for 0.333333 h;Reflux;
Stage #2: with sodium nitrite in water at 0; for 0.25 h;
Stage #3: with hydrogen bromide;copper(I) bromide in water at 0;Heating;
Steps:
4.5. 4-Bromo-2-nitrotoluene (7)
4-Amino-2-nitrotoluene (15.24 g, 99 mmol) was slurried in H2O(125 mL) in a 500 mL 3-necked flask equipped with a condenser,addition funnel and stopper. The suspension was heated to refluxand HBr (48%, 51.5 mL) was added dropwise. The mixture was maintained at reflux for 20 min then cooled to 0 C. A solution ofNaNO2 (6.45 g, 93 mmol) in H2O (40 mL) was added dropwise with rapid stirring while maintaining the temperature at 0 C. The resulting diazonium solutionwas stirred at 0 C for 15 min and then added dropwise to a mechanically stirred mixture of CuBr (15.44 g,108 mmol) in H2O (75 mL) and HBr (48%, 33 mL) cooled to 0e5 Cwith an ice bath. The thick suspension was stirred at rt for 20 min,heated on a steam bath for an additional 20 min and let stand overnight. Steam distillation afforded 4-bromo-2-nitrotoluene (17.79 g, 89%) as yellow prisms: mp 45e45.5 C (lit [20]. mp47 C); 1H NMR (CDCl3) d 8.10 (d, J 2.0, 1 H), 7.62 (dd, J 2.0, 8.2,1 H), 7.24 (d, J 8.2, 1 H), 2.55 (s, 3 H); 13C NMR (CDCl3) d 149.5,136.0, 134.1, 132.6, 127.5, 119.6, 20.1; IR (CDCl3) 3019, 1526, 1350,1215 cm1.
References:
Gribble, Gordon W.;Johnson, Jerry L.;Scott Wiens, P. [Tetrahedron,2021,vol. 85,art. no. 132055]

107650-20-4
153 suppliers
$9.00/250mg

60956-26-5
293 suppliers
$8.00/10g
5437-38-7
444 suppliers
$5.00/10g

60956-26-5
293 suppliers
$8.00/10g

106-38-7
392 suppliers
$14.00/5g

60956-26-5
293 suppliers
$8.00/10g
88-72-2
246 suppliers
$15.00/25g

52414-97-8
111 suppliers
$8.00/1g

60956-26-5
293 suppliers
$8.00/10g