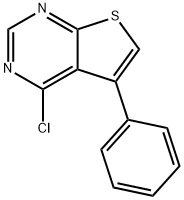
4-CHLORO-5-PHENYLTHIENO[2,3-D]PYRIMIDINE synthesis
- Product Name:4-CHLORO-5-PHENYLTHIENO[2,3-D]PYRIMIDINE
- CAS Number:182198-35-2
- Molecular formula:C12H7ClN2S
- Molecular Weight:246.72
![7-phenyl-9-thia-2,4-diazabicyclo[4.3.0]nona-2,7,10-trien-5-one](/CAS/GIF/35978-39-3.gif)
35978-39-3
65 suppliers
$23.00/1g
![4-CHLORO-5-PHENYLTHIENO[2,3-D]PYRIMIDINE](/CAS/GIF/182198-35-2.gif)
182198-35-2
57 suppliers
$45.00/250mg
Yield: 100%
Reaction Conditions:
with thionyl chloride;N,N-dimethyl-formamide at 80; for 4 h;Product distribution / selectivity;
Steps:
B.5
4-Hydroxyquinazoline (1.0 eq.) was suspended in thionylchloride (2 mL/mmol), three catalytic drops of DMF were added and the mixture was heated to 80°C for 4 h. Thionylchloride was removed in vacuum, toluene (0.5 mL/mmol) was added and removed again under reduced pressure. The remaining solid was dried at high vacuum over night, taken up in ethylene glycol (3 mL/mmol) and treated with 4-(N-Boc-amino)piperidine (1.4 eq.) and DIEA (1.0 eq.). The mixture was heated to 110° for 3 h. For Boc-removal, 4 M HCl/dioxane (3 mL/mmol) was added under cooling (ice bath) and the mixture was stirred at r.t. for 2 h. The solution was partitioned between 1.3 N aq. NaOH / brine (2:1, 20 mL/mmol) and CHCl3 (4x10 mL/mmol). Combined organic phases were dried over MgSO4 to give a yellow-brownish oily solid as crude product.; 1-(5-phenylthieno[2,3-d]pyrimidin-4-yl)piperidin-4-amine Synthesis followed SP5, using 2.3 mmol 5-phenyl-3H-thieno[2,3-d]pyrimidin-4-one or 5-phenyl-thieno[2,3-d]pyrimidin-4-ol to give the title compound quantitatively, containing around 25% water even upon extensive drying in high vacuum.
References:
4SC AG EP1947103, 2008, A1 Location in patent:Page/Page column 18
![7-phenyl-9-thia-2,4-diazabicyclo[4.3.0]nona-2,7,10-trien-5-one](/CAS/GIF/35978-39-3.gif)
35978-39-3
65 suppliers
$23.00/1g
![4-CHLORO-5-PHENYLTHIENO[2,3-D]PYRIMIDINE](/CAS/GIF/182198-35-2.gif)
182198-35-2
57 suppliers
$45.00/250mg
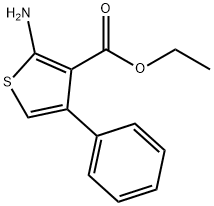
4815-36-5
134 suppliers
$10.00/250mg
![4-CHLORO-5-PHENYLTHIENO[2,3-D]PYRIMIDINE](/CAS/GIF/182198-35-2.gif)
182198-35-2
57 suppliers
$45.00/250mg
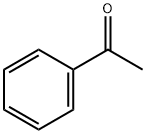
98-86-2
738 suppliers
$9.00/5g
![4-CHLORO-5-PHENYLTHIENO[2,3-D]PYRIMIDINE](/CAS/GIF/182198-35-2.gif)
182198-35-2
57 suppliers
$45.00/250mg
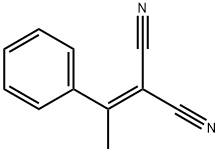
5447-87-0
38 suppliers
inquiry
![4-CHLORO-5-PHENYLTHIENO[2,3-D]PYRIMIDINE](/CAS/GIF/182198-35-2.gif)
182198-35-2
57 suppliers
$45.00/250mg