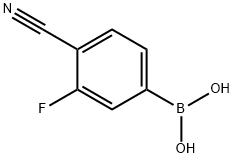
4-CYANO-3-FLUOROPHENYLBORONIC ACID synthesis
- Product Name:4-CYANO-3-FLUOROPHENYLBORONIC ACID
- CAS Number:843663-18-3
- Molecular formula:C7H5BFNO2
- Molecular Weight:164.93
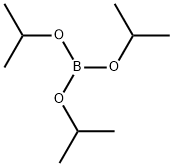
5419-55-6
369 suppliers
$12.00/5g

105942-08-3
372 suppliers
$7.00/5g

843663-18-3
232 suppliers
$8.00/250mg
Yield:843663-18-3 69%
Reaction Conditions:
Stage #1: Triisopropyl borate;4-bromo-6-fluorobenzonitrilewith n-butyllithium in tetrahydrofuran;hexane;toluene at -75 - -74; for 2.25 h;
Stage #2: with hydrogenchloride;water in tetrahydrofuran;hexane;toluene at -20 - 20;Product distribution / selectivity;
Steps:
A
Dissolve 2-Fluoro-4-bromobenzonitrile (200 g, 990 mmol, 1.00 eq.) and triisopropyl borate (228 g, 1188 mmol, 1.2 eq.) in 700 mL of THF and 1400 mL of toluene. Cool the mixture with a dry ice/acetone bath to an internal temperature of -75 0C. Slowly add n-BuLi (396 mL of a 2.5 M solution in hexanes) over a period of 2 hours. After addition is complete, a light-red, thin slurry occurs. Let the solution stir at -74 0C for 15 minutes, allow the solution to warm to -20 0C and then quench with 1500 mL of 2.5 M HCl. Let the solution warm to RT. Separate layers, extract the aqueous layer with EtOAc, dry the combined organic phases with Na2SQ,), filter and concentrate in vacuo to yield a light-brown solid.Triturate the solid with hexane and transfer to a scintered glass funnel. Rinse with hexane one more time to obtain a pale-yellow filtrate. Stir the light-brown solid with cold CH2Cl2 and filter. Rinse with a small volume of CH2Cl2 to yield an off-white solid and a brown filtrate. Dry the solid in a vacuum oven at 40 0C and dried to yield 112g (679 mmol, 69%) of 3-fluoro-4-cyanophenylboronic acid as an off-white solid.
References:
WO2007/87488,2007,A2 Location in patent:Page/Page column 17; 47-48
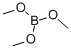
121-43-7
344 suppliers
$14.00/25mL

105942-08-3
372 suppliers
$7.00/5g

843663-18-3
232 suppliers
$8.00/250mg