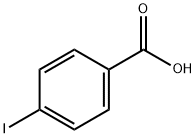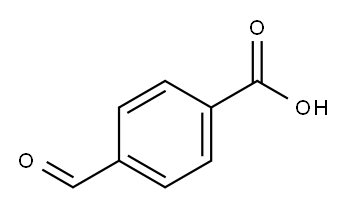
4-Formylbenzoic acid synthesis
- Product Name:4-Formylbenzoic acid
- CAS Number:619-66-9
- Molecular formula:C8H6O3
- Molecular Weight:150.13
Yield:100-21-0 98.3% ,619-66-9 1.4%
Reaction Conditions:
with air;hydrogen bromide;acetic acid;cobalt(II) acetate;manganese(II) acetate at 180; under 25745 Torr; for 0.166667 h;
Steps:
A COMPARATIVE EXAMPLE A
COMPARATIVE EXAMPLE A; Liquid phase oxidation of paraxylene to form TEREPHTHALIC acid ("TA") was conducted without a bromine promoter for comparative purposes. The reactor used was a 71 ML titanium vessel equipped with a thermocouple and a valve for adding gas. During the reaction, the vessel was agitated using mechanical shaking. Heating was provided by mechanically raising a fluidized sand bath to immerse the agitated reactor into THERMOSTATTED sand. The reactor temperature was controlled by adjusting the height of the sand bath relative to the reactor to maintain the temperature indicated by the internal thermocouple. At the completion of the heating cycle, rapid cooling was accomplished by lowering the fluidized sand bath and directing water jets onto the exterior surface of the reactor. The off-gas was vented into a gas sampling bag for analysis and the solid and liquid contents of the reactor were recovered and analyzed. The reactor was charged with a solution of Mn (OAc) 2-4H20 (0.0270 g), Co (OAc) Z-4H20 (0.0265 g), HBr (0.0087 g), and 9.5 g of 95% (by volume) aqueous acetic acid. Before sealing, 0.50 g paraxylene was added. The reactor was sealed and pressurized to 35 KG/CM2 (gauge) with air. The reactor was heated to 180 °C and held at this temperature with agitation for 10 minutes. At the end of this time, the reactor was removed from the heat and cooled quickly to room temperature using a water spray. The contents of the reactor were analyzed. The TA yield was measured and an indication of product quality was determined by the concentration of 4-CBA. Burning ratio was determined as a molar fraction of carbon oxides in the off-gas to paraxylene feed. Concentration of methyl bromide in the off-gas was also measured. The results are reported in Table 1.
References:
WO2005/779,2005,A2 Location in patent:Page 13-14

106-42-3
395 suppliers
$19.00/25ML

619-66-9
411 suppliers
$5.00/5g

106-42-3
395 suppliers
$19.00/25ML
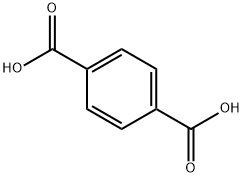
100-21-0
547 suppliers
$6.00/25g

619-66-9
411 suppliers
$5.00/5g

99-94-5
569 suppliers
$9.00/1g

52010-97-6
145 suppliers
$7.00/250mg

100-21-0
547 suppliers
$6.00/25g

619-66-9
411 suppliers
$5.00/5g