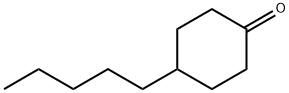
4-Pentylcyclohexanone synthesis
- Product Name:4-Pentylcyclohexanone
- CAS Number:61203-83-6
- Molecular formula:C11H20O
- Molecular Weight:168.28
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

61203-83-6
143 suppliers
$6.00/1g
Yield: 74%
Reaction Conditions:
with 2,2,6,6-tetramethyl-piperidine;n-butyllithium in tetrahydrofuran;hexane at -20 - 20; for 16 h;Inert atmosphere;
Steps:
General procedure for the deprotection of 1,3-dioxolanes promoted by lithium 2,2,6,6-tetramethylpiperidide (Table 2 and Table 3)
General procedure: To a solution of 2,2,6,6-tetramethylpiperidine (TMP) (0.34 mL, 2.00 mmol, 4.0 equiv.) in THF (4 mL) was added n-BuLi (2.4 M in hexane, 0.83 mL, 2.00 mmol, 4.0 equiv.) at -20 or 0 . After stirring for 30 min, a solution of compound 1 or compound 4 (0.50 mmol, 1.0 equiv.) in THF (1 mL) was added. The resulting mixture was stirred before the completion of the reaction (monitored by TLC). Then the reaction mixture was poured into saturated NH4Cl solution (2.5 mL) at 0 and diluted with H2O (5 mL). The layers of the resulting mixture were separated and the aqueous layer was extracted with Et2O (4 × 15 mL). The combined organic layers were dried over Na2SO4, filtered and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel to afford products.
References:
Yuan, Changchun;Yang, Li;Yue, Guizhou;Yu, Tianzi;Zhong, Weiming;Liu, Bo [Tetrahedron Letters,2012,vol. 53,# 51,p. 6972 - 6976] Location in patent:supporting information
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
58479-61-1
401 suppliers
$10.00/25 g

61203-83-6
143 suppliers
$6.00/1g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

58479-61-1
401 suppliers
$10.00/25 g

61203-83-6
143 suppliers
$6.00/1g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![Benzene, 1,1'-[(1,1-dimethylethyl)(ethenyloxy)silylene]bis-](/CAS/20210305/GIF/153711-74-1.gif)
153711-74-1
0 suppliers
inquiry

54410-90-1
85 suppliers
$45.00/50mg

61203-83-6
143 suppliers
$6.00/1g

98789-91-4
0 suppliers
inquiry

61203-83-6
143 suppliers
$6.00/1g