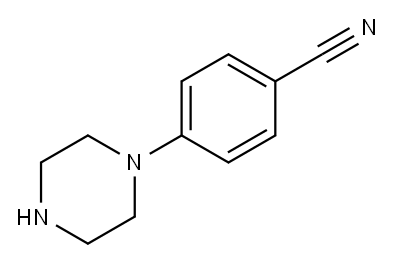
4-Piperazinobenzonitrile synthesis
- Product Name:4-Piperazinobenzonitrile
- CAS Number:68104-63-2
- Molecular formula:C11H13N3
- Molecular Weight:187.24
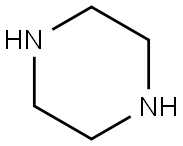
110-85-0
550 suppliers
$5.00/5 g
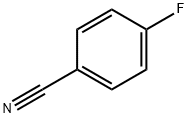
1194-02-1
502 suppliers
$6.00/10g

68104-63-2
129 suppliers
$10.00/250mg
Yield:68104-63-2 95%
Reaction Conditions:
with potassium carbonate in butanone for 96 h;Reflux;
Steps:
6.1.1. 4-(Piperazin-1-yl)benzonitrile (4)
A mixture of 4-fluorobenzonitrile (3, 0.36 g, 3 mmol), piperazine (0.64 g, 7.4 mmol) and K2CO3 (0.85 g, 6.1 mmol) in ethylmethylketone (50 mL) was refluxed for 4 days. The completion of reaction was detected by TLC. The solvent was evaporated under reduced pressure and then chloroform and water was added. The organic layer was extracted. After evaporation of chloroform, the residue was crystallized from chloroform to give compound 4 as a cream solid. Yield: 95%; m.p 82-85 °C; IR (KBr, cm-1): 3325, 2215, 1606. 1H NMR (500 MHz, CDCl3): 7.50 (d, 2H, J = 9.2 Hz, phenyl), 6.86 (d, 2H, J = 9.2 Hz, phenyl), 3.29 (t, 4H, J = 5.2 Hz, piperazine), 3.02 (t, 4H, J = 5.2 Hz, piperazine). MS (ESI): 188 [M + H+]. Anal. Calcd for C11H13N3: C, 70.56; H, 7.0; N, 22.44. Found: C, 70.35; H, 6.82; N, 22.73.
References:
Tahghighi, Azar;Marznaki, Farzane Rezazade;Kobarfard, Farzad;Dastmalchi, Siavoush;Mojarrad, Javid Shahbazi;Razmi, Sepideh;Ardestani, Sussan Kabudanian;Emami, Saeed;Shafiee, Abbas;Foroumadi, Alireza [European Journal of Medicinal Chemistry,2011,vol. 46,# 6,p. 2602 - 2608] Location in patent:experimental part

110-85-0
550 suppliers
$5.00/5 g
623-03-0
441 suppliers
$6.00/25g

68104-63-2
129 suppliers
$10.00/250mg

110-85-0
550 suppliers
$5.00/5 g
623-00-7
466 suppliers
$6.00/10g

68104-63-2
129 suppliers
$10.00/250mg
186650-98-6
69 suppliers
inquiry

68104-63-2
129 suppliers
$10.00/250mg

38212-33-8
153 suppliers
$21.00/1g
57-13-6
697 suppliers
$5.00/5g

68104-63-2
129 suppliers
$10.00/250mg