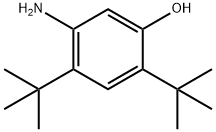
5-AMino-2,4-di-tert-butylphenol synthesis
- Product Name:5-AMino-2,4-di-tert-butylphenol
- CAS Number:873055-58-4
- Molecular formula:C14H23NO
- Molecular Weight:221.34

873055-57-3
67 suppliers
inquiry

873055-58-4
145 suppliers
$40.00/100mg
Yield:873055-58-4 100%
Reaction Conditions:
with ammonium formate;5%-palladium/activated carbon in ethanol for 2 h;Heating / reflux;
Steps:
5-Amino-2,4-di-tert-butyl-phenol
5-Amino-2,4-di-tert-butyl-phenol To a reluxing solution of 2,4-di-tert-butyl-5-nitro-phenol (1.86 g, 7.40 mmol) and ammonium formate (1.86 g) in ethanol (75 mL) was added Pd-5% wt. on activated carbon (900 mg). The reaction mixture was stirred at reflux for 2 h, cooled to room temperature and filtered through Celite. The Celite was washed with methanol and the combined filtrates were concentrated to yield 5-amino-2,4-di-tert-butyl-phenol as a grey solid (1.66 g, quant.). 1H NMR (400 MHz, DMSO-d6) δ 8.64 (s, 1H, OH), 6.84 (s, 1H), 6.08 (s, 1H), 4.39 (s, 2H, NH2), 1.27 (m, 18H); HPLC ret. time 2.72 min, 10-99% CH3CN, 5 min run; ESI-MS 222.4 m/z [M+H]+.
References:
VERTEX PHARMACEUTICALS INCORPORATED US2008/90864, 2008, A1 Location in patent:Page/Page column 8

873055-57-3
67 suppliers
inquiry

7440-44-0
288 suppliers
$12.00/25g

873055-58-4
145 suppliers
$40.00/100mg

873055-55-1
90 suppliers
$60.00/50mg

873055-58-4
145 suppliers
$40.00/100mg

873055-54-0
20 suppliers
inquiry

873055-58-4
145 suppliers
$40.00/100mg
96-76-4
364 suppliers
$6.00/25g

873055-58-4
145 suppliers
$40.00/100mg