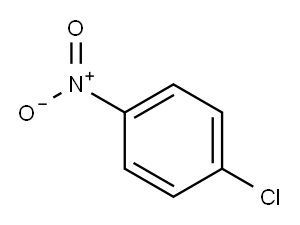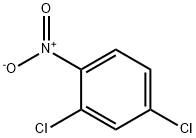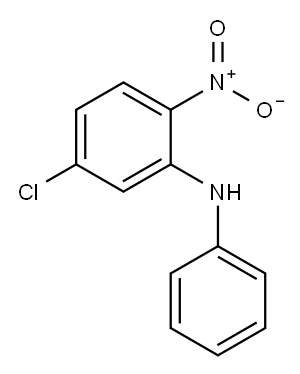
5-CHLORO-2-NITRODIPHENYLAMINE synthesis
- Product Name:5-CHLORO-2-NITRODIPHENYLAMINE
- CAS Number:25781-92-4
- Molecular formula:C12H9ClN2O2
- Molecular Weight:248.67
Yield: 98%
Reaction Conditions:
in dimethyl sulfoxide at 110; for 4 h;
Steps:
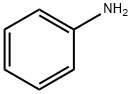
(5-Chloro-2-nitrophenyl)phenylamine A mixture of 4-chloro-2-fluoro-1-nitrobenzene (983 mg, 5.60 mmol) and aniline (1.0 mL, 11.20 mmol) in DMSO (3 mL) was heated at 110° C. for 4 h. After cooling to RT, the reaction mixture was partitioned between EtOAc and water. The organic layer was washed with a saturated aqueous solution of KHSO4 (×3), followed by brine, then dried (Na2SO4) and concentrated in vacuo to afford the title compound as an orange solid (1.37 g, 98%). 1H NMR (CDCl3, 400 MHz): δ 9.54 (1H, s), 8.16 (1H, d, J=9.13 Hz), 7.46 (2H, t, J=7.61 Hz), 7.32-7.22 (3H, m), 7.14 (1H, d, J=2.14 Hz), 6.72 (1H, dd, J=9.12, 2.15 Hz)
References:
Heald, Robert;Price, Stephen;Safina, Brian;Savy, Pascal Pierre Alexandre;Seward, Eileen Mary;Sutherlin, Daniel P.;Waszkowycz, Bohdan US2012/202785, 2012, A1 Location in patent:Page/Page column 147

610-40-2
101 suppliers
$50.00/10g

25781-92-4
181 suppliers
$15.00/1g

610-40-2
101 suppliers
$50.00/10g
62-53-3
631 suppliers
$10.00/1g

25781-92-4
181 suppliers
$15.00/1g