
5-Fluoro-3-methylindole synthesis
- Product Name:5-Fluoro-3-methylindole
- CAS Number:392-13-2
- Molecular formula:C9H8FN
- Molecular Weight:149.16
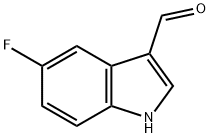
2338-71-8
160 suppliers
$15.00/250mg

392-13-2
128 suppliers
$32.00/100mg
Yield: 82.3%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at 70; for 2 h;
Steps:
58.1 Step 1) Synthesis of 5-fluoro-3-methyl-1H-indole
5-Fluoro-1H-indole-3-carbaldehyde (3.2 g, 19.6 mmol) was dissolved in 10 mL of anhydrous tetrahydrofuran, then lithium aluminium hydride (2.61 g, 68.6 mmol) was added to the solution. The mixture was reacted at 70 °C for 2 hours. After the reaction, the reaction mixture was cooled to RT and quenched with aqueous sodium hydroxide (18 mL, 2 0%). 60 mL of dichloromethane was added to the mixture and the resulting mixture was washed with saturated aqueous sodium chloride (40 mL). The organic layer was dried over anhydrous sodium sulfate and filtered. The filtrate was concentrated in vacuo and the residue was purified by silica gel chromatography eluted with PE/EA(V/V)=50/1 to give the title compound as a light yellow solid (2.41 g, 82.3%). The compound was characterized by the following spectroscopic data: MS (ESI, pos. ion) m/z:150.2 [M+Hfb; and ‘H NMR (400 MHz, CDC13): 6 7.30-7.23 (m, 2H), 7.04 (s, 1H), 6.97 (td, J = 9.0, 2.0 Hz, 1H), 2.33 (s, 3H).
References:
SUNSHINE LAKE PHARMA CO., LTD.;ZHANG, Yingjun;JIN, Chuanfei;ZHONG, Wenhe;ZHANG, Ji;GAO, Li;CHEN, Kangzhi WO2015/90233, 2015, A1 Location in patent:Paragraph 00362
67-56-1
733 suppliers
$7.29/5ml-f

399-52-0
358 suppliers
$10.00/1g

392-13-2
128 suppliers
$32.00/100mg

399-52-0
358 suppliers
$10.00/1g

392-13-2
128 suppliers
$32.00/100mg
371-14-2
72 suppliers
inquiry

392-13-2
128 suppliers
$32.00/100mg
823-85-8
360 suppliers
$5.00/5g
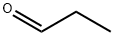
123-38-6
421 suppliers
$14.00/5mL

392-13-2
128 suppliers
$32.00/100mg