
5-Hydroxymethylfurfural synthesis
- Product Name:5-Hydroxymethylfurfural
- CAS Number:67-47-0
- Molecular formula:C6H6O3
- Molecular Weight:126.11
Continuous synthesis of 5-hydroxymethylfurfural from glucose using a combination of AlCl3 and HCl as catalyst in a biphasic slug flow capillary microreactor

492-61-5
136 suppliers
$32.00/10g

67-47-0
570 suppliers
$5.00/100mg
Yield:67-47-0 100%
Reaction Conditions:
with formic acid;tetrabutylammomium bromide in ethanol;water at 270; under 112511 Torr; for 4 h;Pressure;Reagent/catalyst;
Steps:
3
General procedure: The reaction, the reaction was carried out using an apparatus as shown in Figure 1. The reactor, temperature controlled reactor 1 (SUS316 made an inner diameter of 0.9cm × 15cm long with a heater (capacity 9.53cm3), same reactor 2 (reaction tube length is temperature controlled by the heater (SUS316 made inner diameter 0. 1 cm × length 500 cm (volume 3.92cm3)), condenser and using the apparatus consisting of back pressure valve. filled with crude pulp (0.9245g) in the reactor 1, 0.1% formic acid using a pump the aqueous solution 15 MPa, 225 ° C., flowed at 0.5 mL / min were introduced into the reactor 2 while only the cellulose component contained in the crude pulp were extracted with an acid saccharification. Also 0.1M tetrabutylammonium bromide solution using a pump (ethanol: water = 1: 1) to 15MPa conditions, heated to 280 ° C. with a heater, mixed with saccharified solution of the previous pulp T-micromixer , after the temperature of the solution after mixing in the rapidly 270 , it was allowed to react at 270 as it is in the reactor 2. Acid saccharification + extraction time in the reactor 1 at this time (residence time), 1.90 seconds, the reaction time in the reactor 2 (residence time) is 0.79 seconds, a total of 2.7 seconds. After the reaction is cooled in a cooler to lower the pressure to atmospheric pressure at the back pressure valve to obtain 5-hydroxy-furfural-containing aqueous solution. HMF The resulting aqueous solutions were sampled every 20 minutes (100 mL), and immediately the content of HMF contained sample was analyzed by liquid chromatography (input condition). The resulting weight yield of HMF after 240 minutes 71.6% (Figure 2, Table 1), and the crude residue pulp in the reactor 1 is almost no near 100% was converted. Furthermore, the selectivity of each sample, 71.5% and 100% (Table 1). Using the same reaction apparatus as in Example 1, raw material with glucose, catalyst using tetrabutylammonium bromide, saw a change in the yield of HMF by the pressure effect (Figure 9).As a result, as a solvent, water: ethanol = 50: when the 50, equal to or greater than 15MPa, HMF yield is almost 100% next to the also,, 60: in the case of 40, at 10MPa, of almost 100% to obtain a yield, 100: 0 for (water only) was 30%, even 15 MPa.
References:
JP2015/209411,2015,A Location in patent:Paragraph 0003; 0058-0059; 0063
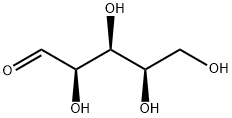
58-86-6
424 suppliers
$5.00/25g

67-47-0
570 suppliers
$5.00/100mg
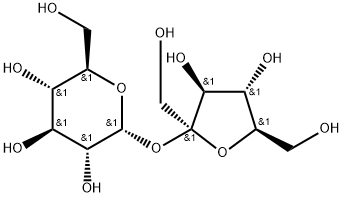
57-50-1
564 suppliers
$5.00/10g

67-47-0
570 suppliers
$5.00/100mg

41579-20-8
0 suppliers
inquiry

67-47-0
570 suppliers
$5.00/100mg
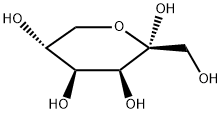
87-81-0
336 suppliers
$12.50/5g

67-47-0
570 suppliers
$5.00/100mg