
4-Nitrophthalonitrile synthesis
- Product Name:4-Nitrophthalonitrile
- CAS Number:31643-49-9
- Molecular formula:C8H3N3O2
- Molecular Weight:173.13
Synthesis of 4-Nitrophthalonitrile:SOCl2 (83.5 mL. 1.144 mol) was added dropwise under nitrogen purge to dry DMF (200 mL) which had been cooled to 0-5 °C. The solution was allowed to stir for 15 min at 0-5 °C. The 4-nitrophthalamide (60.1 g, 0.286 mol) was then added and the solution was allowed to slowly warm to room temperature and react for 18 h under nitrogen purge. The solution was then slowly added to ice water to crystallize and precipitate the product. The 4-nitrophthalonitrile was collected using vacuum filtration, washed with ice cold water, and allowed to air dry overnight; yield: 45.2 g (92%); m.p.: 141 °C (det. by DSC)

13138-53-9
119 suppliers
$9.00/1g

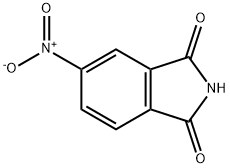
31643-49-9
435 suppliers
$6.00/5g
Yield:31643-49-9 89%
Reaction Conditions:
with thionyl chloride in N,N-dimethyl-formamide at 5; for 18 h;Inert atmosphere;
Steps:
2.4 Synthesis of 4-nitrophthalonitrile (3)
The thionyl chloride (286mol) was slowly added to 50mL of N,N-dimethylformamide at a temperature lower than 5°C, was stirred in an argon atmosphere. 15g of 4-nitrophthalamide was added and the reaction was maintained for 18h. The crude product was precipitated from a mixture of water-ice [1]. Yield 89%; 1H NMR (300MHz, DMSO-d6; δ: ppm): 8.44 (d, J=8.7, 1H, H-Ar); 8.68 (dd, J=2.1Hz, J=8.4Hz, 1H, H-Ar); 9.04 (d, J=2.1, 1H, H-Ar). 13C NMR (75MHz, DMSO-d6, δ): 114.55; 114.86; 116.60; 120.22; 128.53; 128.81; 135.27; 149.70. FTIR: 3090 (C-H aromatic); 2245 (CN); 1534 (asymmetric N-O band); 1351 (symmetric N-O band); 852 (C-N).
References:
Kahouech;Hriz;Touaiti;Bassem [Materials Research Bulletin,2016,vol. 75,p. 144 - 154]
89-40-7
316 suppliers
$5.00/5g

31643-49-9
435 suppliers
$6.00/5g
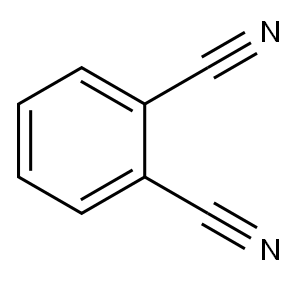
91-15-6
251 suppliers
$13.00/50g

31643-49-9
435 suppliers
$6.00/5g
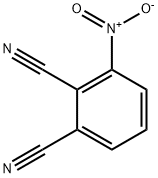
51762-67-5
374 suppliers
$5.00/1g

136918-14-4
0 suppliers
inquiry

31643-49-9
435 suppliers
$6.00/5g

610-27-5
408 suppliers
$6.00/5g

31643-49-9
435 suppliers
$6.00/5g