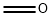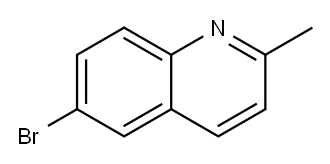
6-Bromo-2-methylquinoline synthesis
- Product Name:6-Bromo-2-methylquinoline
- CAS Number:877-42-9
- Molecular formula:C10H8BrN
- Molecular Weight:222.08
Yield:877-42-9 81%
Reaction Conditions:
with Ag(I) exchanged montmorillonite K10 in neat (no solvent) at 120; for 3 h;Green chemistry;Doebner-von Miller Reaction;
Steps:
General procedure for the synthesis of quinoline derivatives
General procedure: Amine (1.50 mmol) and aldehyde (1.00 mmol) were dissolved in 1.5 mL of Et2O in a reaction vial equipped with a magnetic stirrer bar, followed by the addition of Ag(I)-exchanged Montmorillonite K10 (0.50 g). After 5 min stirring, the solvent was removed in vacuo to obtain a dry powder. The reaction mixture was heated at a temperature of 120 oC for 3 h. After cooling to room temperature, the reaction mixture was filtered through a short silica plug and the solid residues washed well with CH2Cl2. The solvent was removed in vacuo and the crude product purified by column chromatography over silica gel eluting with a mixture of Hexane EtOAc to produce the title compounds (3a-3k). NOTE. For the recycle/reuse study, the crude product was gravity filtered and the clay material washed several times with CH2Cl2, air dried and weighed. This process was repeated for consecutive recycle/reuse reactions.
References:
Jayram, Janeeka;Jeena, Vineet [Heterocycles,2016,vol. 92,# 12,p. 2213 - 2224]
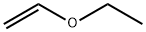
109-92-2
265 suppliers
$16.00/25mL
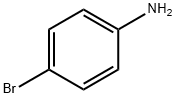
106-40-1
456 suppliers
$12.00/5g

877-42-9
189 suppliers
$16.00/1g