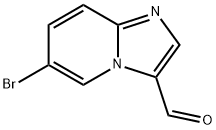
6-BROMOIMIDAZO[1,2-A]PYRIDINE-3-CARBALDEHYDE synthesis
- Product Name:6-BROMOIMIDAZO[1,2-A]PYRIDINE-3-CARBALDEHYDE
- CAS Number:30384-96-4
- Molecular formula:C8H5BrN2O
- Molecular Weight:225.04

1072-97-5
697 suppliers
$9.00/1g

2065-75-0
319 suppliers
$6.00/5g
![6-BROMOIMIDAZO[1,2-A]PYRIDINE-3-CARBALDEHYDE](/CAS/GIF/30384-96-4.gif)
30384-96-4
91 suppliers
$13.00/100mg
Yield: 80%
Reaction Conditions:
in ethanol;water at 110; for 0.166667 h;Inert atmosphere;Microwave irradiation;Green chemistry;Solvent;Temperature;Time;
Steps:
General procedure for the synthesis of imidazo[1,2-a]pyridine-3-carbaldehyde (3)
General procedure: 6-bromo-2-aminopyridine (0.208 g, 1.2 mmol, 1 eq.) and 2-bromomalonaldehyde (0.272 g, 1.8 mmol, 1.5 eq.) were dissolved in the mixture of ethanol and water (v/v 1:1, total 2 ml) and placed in the pressure vial, equipped with a magnetic bar. The mixture was stirred for 1 min and purge with argon via syringe. Then microwave (MW) irradiation (with initial 150 W power) was applied. Depending on the substituents present in 2-aminopyridine 1, the following conditions were applied: a) for 2-aminopyridines substituted with halogen, or nitro or cyano groups: 10 min, 110 °C; or b) for 2-aminopyridines substituted with methyl or phenyl groups: 20 min, 100 °C. Afterwards, the reaction mixture was evaporated. Next the reaction mixture was neutralized with TEA, diluted with DCM (10 mL), and adsorbed on silica gel (~3 g).* The solvent was evaporated, and the residue was subjected to column chromatography using gradient DCM/acetone (100:0 => 75:25) as eluent to give product as yellow powder.
References:
Kusy, Damian;Maniukiewicz, Waldemar;Błażewska, Katarzyna M. [Tetrahedron Letters,2019,vol. 60,# 45,art. no. 151244] Location in patent:supporting information
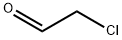
107-20-0
4 suppliers
$18.10/5ml

138888-98-9
19 suppliers
inquiry
![6-BROMOIMIDAZO[1,2-A]PYRIDINE-3-CARBALDEHYDE](/CAS/GIF/30384-96-4.gif)
30384-96-4
91 suppliers
$13.00/100mg