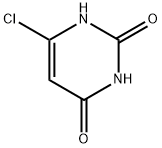
6-Chlorouracil synthesis
- Product Name:6-Chlorouracil
- CAS Number:4270-27-3
- Molecular formula:C4H3ClN2O2
- Molecular Weight:146.53
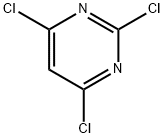
3764-01-0
400 suppliers
$6.00/5g

4270-27-3
309 suppliers
$7.00/5g
Yield:4270-27-3 97%
Reaction Conditions:
Stage #1:2,4,6-trichloropyrimidine with sodium hydroxide in water for 1 h;Reflux;
Stage #2: with hydrogenchloride in water; pH=2 - 3
Steps:
4.3.2. 6-Chlorouracil (6{1,1})
A solution of 2,4,6-trichloropyrimidine (11) (10.21 g, 0.56 mol) in water (100 mL) containing NaOH (8.9 g, 0.22 mol) was stirred under reflux for 1 h. The reaction mixture was adjusted to pH 2-3 with conc. aqueous HCl and was kept in a refrigerator overnight. The precipitate was collected by filtration, washed with cold water and dried in vacuo over phosphorus pentoxide. 6.43 g (0.54 mol, 97%) of 6-clorouracil (6{1,1}) were obtained as a white crystalline solid. Spectroscopic data was identical with those reported in the literature [28] and [40]. m.p. >280 °C. 1H NMR (300 MHz, DMSO-d6) δ (ppm): 12.00 (br s, 1H, NH), 11.09 (br s, 1H, NH), 5.66 (s, 1H, CH). IR (KBr, ν cm-1): 3095 (N-H), 1729, 1709, 1652 (CO), 1616 (CC), 1380 (C-N), 841 (N-H), 790 (C-Cl).
References:
Puig-De-La-Bellacasa, Raimon;Gimenez, Laura;Pettersson, Sofia;Pascual, Rosalia;Gonzalo, Encarna;Este, Jose A.;Clotet, Bonaventura;Borrell, Jose I.;Teixido, Jordi [European Journal of Medicinal Chemistry,2012,vol. 54,p. 159 - 174] Location in patent:experimental part
6320-15-6
178 suppliers
$18.00/250mg

4270-27-3
309 suppliers
$7.00/5g
67-52-7
466 suppliers
$27.30/25g

4270-27-3
309 suppliers
$7.00/5g