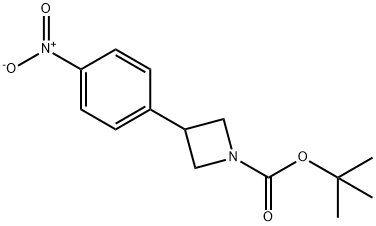
tert-Butyl 3-(4-nitrophenyl)azetidine-1-carboxylate synthesis
- Product Name:tert-Butyl 3-(4-nitrophenyl)azetidine-1-carboxylate
- CAS Number:883901-62-0
- Molecular formula:C14H18N2O4
- Molecular Weight:278.3
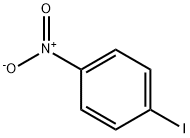
636-98-6
18 suppliers
$17.00/5g
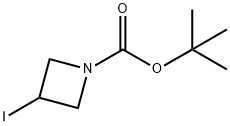
254454-54-1
247 suppliers
$15.00/1g

883901-62-0
24 suppliers
inquiry
Yield:883901-62-0 72%
Reaction Conditions:
Stage #1: tert-butyl-3-iodoazetidine-1-carboxylatewith chloro-trimethyl-silane;ethylene dibromide in tetrahydrofuran at 20 - 80; for 2.5 h;Inert atmosphere;
Stage #2: 4-Iodonitrobenzenewith tris-(dibenzylideneacetone)dipalladium(0);trifuran-2-yl-phosphane in tetrahydrofuran at 55; for 3 h;
Steps:
(a) tert-Butyl-3-(4-nitrophenyl) azetidine- 1-carboxylate (16)
(a) tert-Butyl-3-(4-nitrophenyl) azetidine- 1-carboxylate (16)1,2-Dibromoethane (0.146 mL, 1.69 mmol) was added to a vigorously stirred suspension of zinc dust (0.901 g, 13.8 mmol) in THF (3.5 mL) under a nitrogen atmosphere and the resulting suspension heated at 80 00 for 10 minutes. Trimethylsilyl chloride (0.202 mL, 1.59 mmol) in THF (1.75 mL) was added at roomtemperature and after stirring for 4 minutes a solution of tert-butyl 3-iodoazetidine-1- carboxylate (3.00 g, 10.6 mmol) in THF (3.5 mL) was added dropwise over a period of 15 minutes. The resulting mixture was stirred at room temperature for 2 hours then Pd2(dba)3 (0.155 g, 0.170 mmol) and tri-2-furylphosphine (0.143 g, 0.615 mmol) were added followed by 1-iodo-4-nitrobenzene (2.90 g, 11.7 mmol) in THF (18 mL). Theresulting mixture was heated at 55 00 for 3 hours then quenched at room temperature with a saturated aqueous sodium chloride solution (15 mL). The aqueous phase was extracted with DCM (2 x 15 mL) then the combined organic fractions were dried (magnesium sulfate), filtered and evaporated in vacuo. The residue was purified using silica gel column chromatography (CombiFlash Rf, 40 gSi02 Cartridge, 10-40% EtOAc in cyclohexane) to give the title compound 16 as anorange oil (2.14 g, 72%); 1H NMR (300 MHz, CDCI3) O 8.24 (dd, J= 6.8, 1.9 Hz, 2H),7.51 (d, J= 8.6 Hz, 2H), 4.41 (t, J= 8.7 Hz, 2H), 3.98 (dd, J= 8.5, 5.7 Hz, 2H), 3.89-3.81 (5, 1H), 1.49 (5, 9H).
References:
WO2014/26243,2014,A1 Location in patent:Page/Page column 88