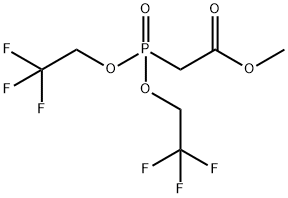
BIS(2,2,2-TRIFLUOROETHYL) (METHOXYCARBONYLMETHYL)PHOSPHONATE synthesis
- Product Name:BIS(2,2,2-TRIFLUOROETHYL) (METHOXYCARBONYLMETHYL)PHOSPHONATE
- CAS Number:88738-78-7
- Molecular formula:C7H9F6O5P
- Molecular Weight:318.11
Yield:88738-78-7 94%
Reaction Conditions:
Stage #1:methyl 2-(bis((trimethylsilyl)oxy)phosphoryl)acetate with iodine;triphenylphosphine in chloroform at 20; for 0.25 h;Inert atmosphere;
Stage #2: with 1H-imidazole in chloroform at 20 - 50; for 0.75 h;Inert atmosphere;
Stage #3:2,2,2-trifluoroethanol in chloroform at 60; for 5 h;Inert atmosphere;
Steps:
Methyl Bis(2,2,2-trifluoroethyl)phosphonoacetate (Still-Gennari reagent, 1)
TMSBr (90 L, 0.691 mmol) was added at r.t. to a solution of trimethyl phosphonoacetate (2; 50.3mg, 0.276 mmol) in anhydrous CH2Cl2 (0.55 mL). After stirring at r.t. for 5 h under argon,evaporation of the reaction mixture in vacuo gave methyl2-{bis[(trimethylsilyl)oxy]phosphoryl}acetate (4), which was used without further purification.Ph3P (181 mg, 0.691 mmol) and I2 (175 mg, 0.691 mmol) were added to a solution of 4 in anhydrous CHCl3 (1.8 mL) at r.t. under argon. After stirring at r.t. for 15 min under argon, imidazole(188 mg, 2.76 mmol) was added. The reaction mixture was stirred for 15 min at r.t. and then for 30min at 50 °C. Afterwards, 2,2,2-trifluoroethanol (79 l, 1.10 mmol) was added and the reactionmixture was stirred at 60 °C for 5 h. After filtration of the reaction mixture, the filtrate wasevaporated in vacuo to give a crude product 1, which was purified by column chromatography[Silica Gel PSQ 60B: n-hexane-EtOAc (2:1)] to afford 1 (82.3 mg, 94%) as a colorless oil. IR (neat) 1747, 1265, 1174, 1072, 963 cm-1. 1H NMR (500 MHz, CDCl3) δ = 4.51-4.42 (m, 4H),3.78 (s, 3H), 3.17 (d, 2JH,P = 21.1 Hz, 2H). 13C NMR (125 MHz, CDCl3) δ = 165.2 (d, 2JC,P = 4.5Hz), 122.5 (qd, 1JC,F = 277.1 Hz, 3JC,P = 8.2 Hz), 62.7 (qd, 2JC,F = 38.2 Hz, 2JC,P = 5.5 Hz), 53.1,33.8 (d, 1JC,P = 145.1 Hz). HRMS (ESI): m/z [M + Na]+ calcd for C7H9F6O5PNa: 340.9990; found:340.9982. Anal. Calcd for C7H9F6O5P: C, 26.43; H, 2.85. Found: C, 26.28; H, 2.89.
References:
Sano, Shigeki;Matsumoto, Tomoya;Toguchi, Munehisa;Nakao, Michiyasu [Synlett,2018,vol. 29,# 11,art. no. ST-2018-U0114-L,p. 1461 - 1464] Location in patent:supporting information
![Acetic acid, 2-[bis[(trimethylsilyl)oxy]phosphinyl]-, methyl ester](/CAS/20210111/GIF/67605-34-9.gif)

