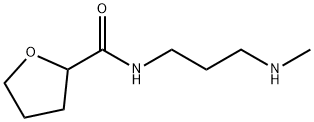
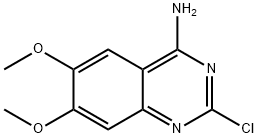
Alfuzosin synthesis
- Product Name:Alfuzosin
- CAS Number:81403-80-7
- Molecular formula:C19H27N5O4
- Molecular Weight:389.45
Yield:81403-80-7 80%
Reaction Conditions:
in i-Amyl alcohol at 25 - 128; for 12 h;Heating / reflux;
Steps:
3
Example 3:(+/-)-N-[3-[(4-amino-6,7-dimethoxy-2-quinazolinyl)methylamino]propyl]tetrah.ydro-2- furancarboxamide: 2-Chloro-4-amino-6,7-dimethoxyquinazoline (30gm, 0.125 mole) is stirred with iso amyl alcohol (300 ml) at 25-3O0C. Then tetrahydro-N-[3-(methylamino)-propyl]-2- furancarboxamide (26.73gm, 0.15 mole) is added to this mixture and reaction mixture is heated to reflux at 127-1280C for 12 hours. After the completion of reaction, the reaction mixture is cooled to 25-3O0C. Then the reaction mixture is further cooled to 8-1O0C and reaction mixture is basified to pH 10-11 using 20% aqueous sodium hydroxide solution. Then the aqueous phase is extracted with ethyl acetate (100 ml x 3). The combined ethyl acetate layer is then washed with water (100 ml) and dried over anhydrous sodium sulphate. The solvent is then removed under the reduced pressure at 45-5O0C. Then hexane (90 ml) is added to the above residue obtained and stirred at 25-3O0C. The solid product obtained is filtered and dried at dried at 50-550C for 15 hours. (40gm, 80 %). Melting Range: 182-1840C Mass: [M+l]+ = 390.81HNMR (CDCl3): delta 8.63 (lH,s), 6.98 (lH,s), 6.82 (lH,s) 5.84 (2H,s), 4.51-4.46 (lH,dd), 4.1-3.9 (10H,m), 3.62-3.54 (lH,mH 3.4-3.33 (lH,m), 3.16 (3H,s), 3-2.86 (lH,m), 2.33-2.2 (2H,m), 2-1.17 (3H,m), 1.64-1.55 (lH,m).
References:
UNICHEM LABORATORIES LIMITED WO2008/84493, 2008, A2 Location in patent:Page/Page column 9

81403-68-1
365 suppliers
$28.00/25mg

81403-80-7
129 suppliers
inquiry

76362-29-3
29 suppliers
inquiry

81403-80-7
129 suppliers
inquiry

16874-33-2
282 suppliers
$6.00/5g

81403-69-2
37 suppliers
$150.00/25 mg

81403-80-7
129 suppliers
inquiry