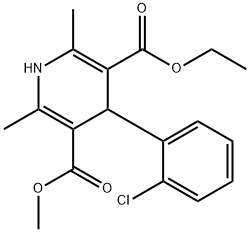
Amlodipine Impurity 48 synthesis
- Product Name:Amlodipine Impurity 48
- CAS Number:39562-06-6
- Molecular formula:C18H20ClNO4
- Molecular Weight:349.81

141-97-9
773 suppliers
$5.00/25g

89-98-5
634 suppliers
$16.00/25g

21731-17-9
60 suppliers
inquiry

39562-06-6
17 suppliers
inquiry
Yield:39562-06-6 26%
Reaction Conditions:
in ethanol;acetic acid at 20 - 95; for 3 h;
Steps:
56
Ethyl acetoacetate (638 μL, 99%, 5.00 mmol), 2-chlorobenzaldehyde (562 μL, 99%, 5.00 mmol) and methyl-3-aminocrotonate (593 mg, 97%, 5.00 mmol) were taken up in EtOH (3.25 mL) at rt. AcOH (217 μL) was added and the mixture was heated to 95 0C. After 3h, the reaction mixture was cooled to ambient temperature, diluted with EtOAc (20 mL), dried over Na2SO4 and crystallized from EtOAc/hexane (1 :9) to afford 451 mg (26%) of 3-ethyl 5-methyl 4-(2-chlorophenyl)-l,4-dihydro-2,6-dimethylpyridine-3,5-dicarboxylate as a white solid: MP 125-127 0C; 1H NMR (500 MHz, CDCl3) δ 1.20 (t, J= 7.0 Hz, 6H), 2.30 (s, 3H), 2.31 (s, 3H), 3.61-3.62 (m, 3H), 4.05-4.10 (m, 4H), 5.40 (s, IH), 5.70-5.74 (m, IH), 7.02-7.06 (m, IH), 7.10-7.15 (m, IH), 7.22-7.25 (m, IH), 7.35-7.39 (m, IH); 13C NMR (125 MHz, CDCl3) δ 14.3, 19.4, 19.5, 19.6, 37.2, 37.3, 37.6, 50.8, 50.9, 59.8, 103.8, 103.9, 104.1,126.7, 126.8, 126.9, 127.3, 129.3, 131.2, 131.4, 131.6, 132.4, 143.9, 144.0, 144.1, 145.6,145.8, 145.9, 167.6, 167.7, 168.0, 168.1; MS (ES) m/z 372 (M+Na)+, 350 (M+H)+, 318, 304, 272, 238; m/z 350.098 (calcd for Ci8H2iClNO4 (M+H)+: 350.115).
References:
WO2008/6070,2008,A2 Location in patent:Page/Page column 150-151