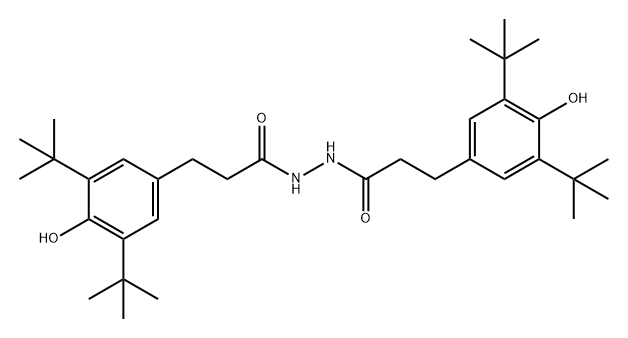
antioxidant 1024 synthesis
- Product Name:antioxidant 1024
- CAS Number:32687-78-8
- Molecular formula:C34H52N2O4
- Molecular Weight:552.79
(1) Dissolve 54 g of hydrazine hydrate in 600 mL of a mixed solution of methanol and ethanol (V methanol: V ethanol = 1:2), then transfer to a dry three-necked flask, and then add 76 g of β-(3, 5-di-tert-butyl-4-hydroxyphenyl) methyl propionate, the three-necked flask is fixed on the iron stand, the ultrasonic time is 10min at room temperature, the intensity is 50KHz, and then the stirrer is placed, and at room temperature , Stir and react for 12h in a nitrogen protected environment, and wait until the reaction liquid is white and viscous;
(2) The white viscous reaction liquid obtained in step (1) is subjected to vacuum distillation. When a large amount of solids are deposited, the vacuum distillation is stopped, and then water is added to the reaction flask and stirred, that is, white crystals are deposited. After suction filtration , Recrystallize with n-hexane to obtain pure β-(3,5-di-tert-butyl-4-hydroxyphenyl) propionyl hydrazide.
2. The synthesis steps of antioxidant 1024
(1) First, add 18 g of anhydrous β-(3,5-di-tert-butyl-4-hydroxyphenyl) propionyl hydrazide, 10 g of ethyl acetoacetate, and 75 mL of dehydrated methanol and toluene into a three-necked flask. Solution (V methanol:V toluene=1:80), then add the rotor, and fix the thermometer, reflux condenser and nitrogen protection device on the three-necked flask;
(2) Place the three-necked flask containing the reactants in step (1) in an oil bath to slowly raise the temperature, and stop the reaction after reacting for 3 hours at 85°C;
(3) Cool the reaction solution of step (2) until crystals are precipitated, and then continue to drop to room temperature until the crystals are completely precipitated, and then perform suction filtration to obtain crystals. Wash the obtained crystals with water 3 times, and then use 5% hydrochloric acid Rinse, finally wash with water to neutrality, filter, and dry to obtain the final product Antioxidant 1024.
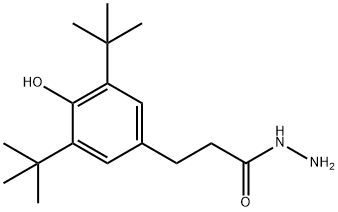
32687-77-7
34 suppliers
inquiry

32687-78-8
216 suppliers
$14.00/5g
Yield:32687-78-8 98.8%
Reaction Conditions:
with ethyl acetoacetate in methanol;toluene at 85; for 3 h;Inert atmosphere;Temperature;
Steps:
4.2.1; 4.2.2 2. Synthesis steps of N,N'-bis[β(3,5-di-tert-butyl-4-hydroxyphenyl)propionyl]indole
(1) First, 18 g of anhydrous β-(3,5-di-tert-butyl-4-hydroxyphenyl)propionylhydrazide, 10 g, was placed in a three-necked flask.Ethyl acetoacetate and 75 mL of a mixed solution of methanol and toluene (V methanol: V toluene = 1:80), and then added to the rotor, and a thermometer, a reflux condenser and a nitrogen protection device were fixed on the three-necked flask;(2) The three-necked flask containing the reactant in the step (1) is slowly heated under an oil bath condition at 85 ° C.Next, the reaction was stopped after 3 hours of reaction;(3) cooling the reaction liquid of the step (2) until crystals are precipitated, and then continuing to fall to room temperature until the crystals are completely precipitated.Filtration was carried out to obtain crystals, and the obtained crystals were washed 3 times with water, then rinsed with 5% hydrochloric acid, and finally washed with water until neutral, filtered, and dried to obtain a final product.The final product yield was 98.8% and the purity was 98.5%.
References:
CN108484434,2018,A Location in patent:Paragraph 0041; 0042; 0043; 0044; 0050; 0051; 0052; 0053
6386-38-5
165 suppliers
$13.00/10g

32687-78-8
216 suppliers
$14.00/5g