
AZ20 synthesis
- Product Name:AZ20
- CAS Number:1233339-22-4
- Molecular formula:C21H24N4O3S
- Molecular Weight:412.51
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
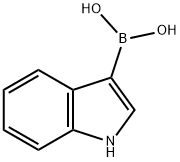
220465-43-0
163 suppliers
$34.00/1 g

1233339-22-4
109 suppliers
$25.00/1mg
Yield: 51%
Reaction Conditions:
with sodium carbonate;bis-triphenylphosphine-palladium(II) chloride in 1,2-dimethoxyethane;water at 90;
Steps:
3.01
Example 3.014-{4-r(3R)-3-Methylmorpholin-4-yl1-6-ri-(methylsulfonyl)cvclopropyl1pyrimidin-2-yl}- lH-indoleBis(triphenylphosphine)palladium chloride (1.692 g, 2.41 mmol), (R)-4-(2-chloro-6-(l- (methylsulfonyl)cyclopropyl)pyrimidin-4-yl)-3-methylmorpholine (8.00 g, 24.11 mmol), IH- indol-4-ylboronic acid (4.27 g, 26.52 mmol) and 2M aqueous sodium carbonate (36.2 rnL, 72.33 mmol) were suspended in DME:water 4:1 (170 mL) and heated to 90 0C overnight. The DME was removed and the reaction mixture diluted with EtOAc (100 mL). The mixture was washed with water (2 x 100 mL), the organics separated, filtered through a pad of Celite and concentrated in vacuo on to silica. The residue was purified by chromatography on silica with an elution gradient of 0 to 10% EtOAc in DCM. Fractions containing product were combined and evaporated. The residue was purified by chromatography on silica eluting with a gradient of 0-25% EtOAc in DCM. Fractions containing product were combined and evaporated onto reverse phase Cl 8 silica. The crude product was purified by reverse phase using a 415g HP Cl 8 column using decreasingly polar mixtures of water (containing 1% NH3) and MeCN as eluents. Fractions containing product were combined and evaporated. The residue was taken up in dry MeOH and dried over MgSO4. The mixture was filtered and the solvent evaporated, leaving a gum. The gum was dissolved in DCM (500 mL), filtered and the solvent removed under reduced pressure. The residue was dissolved in MeOH (50 mL) and allowed to stir at RT overnight. The resultant precipitate was collected by filtration to afford the title compound (5.1O g, 51%); 1H NMR (400 MHz, DMSO-/): 1.29 (3H, d), 1.57 - 1.64 (2H, m), 1.68 - 1.78 (2H, m), 3.24-3.31 (IH, td), 3.29 (3H, s), 3.51 (IH, td), 3.67 (IH, dd), 3.80 (IH, d), 3.93 - 4.06 (IH, dd), 4.21 (IH, d), 4.61 (IH, bs), 6.85 (IH, s), 7.21 (IH, t), 7.32 (IH, t), 7.46 (IH, t), 7.56 (IH, d), 8.06 (IH, dd), 11.25 (IH, s); m/z: (ESI+) MH+, 413.12. Chiral HPLC: (HPI lOO System 4, 5μm Chiralpak AS-H (250mm x 4.6mm) column eluting with iso- Hexane/EtOH/TEA 60/40/0.1) Rf, 8.815 >99%.
References:
ASTRAZENECA AB;ASTRAZENECA UK LIMITED;FOOTE, Kevin, Michael;NISSINK, Johannes, Wilhelmus, Maria WO2010/73034, 2010, A1 Location in patent:Page/Page column 68-69
![Morpholine, 4-[2-chloro-6-[(methylsulfonyl)methyl]-4-pyrimidinyl]-3-methyl-, (3R)-](/CAS/20210305/GIF/1233339-73-5.gif)
1233339-73-5
0 suppliers
inquiry

1233339-22-4
109 suppliers
$25.00/1mg

1233339-71-3
5 suppliers
inquiry

1233339-22-4
109 suppliers
$25.00/1mg
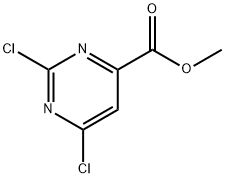
6299-85-0
180 suppliers
$10.00/1g

1233339-22-4
109 suppliers
$25.00/1mg

1233339-69-9
9 suppliers
inquiry

1233339-22-4
109 suppliers
$25.00/1mg