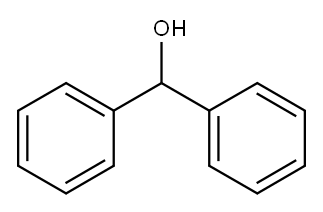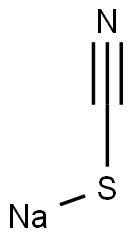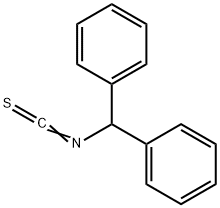
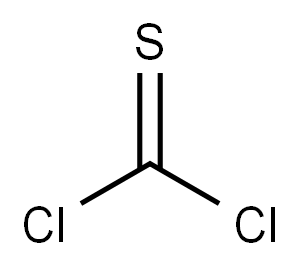
BENZHYDRYL ISOTHIOCYANATE synthesis
- Product Name:BENZHYDRYL ISOTHIOCYANATE
- CAS Number:3550-21-8
- Molecular formula:C14H11NS
- Molecular Weight:225.31
463-71-8
139 suppliers
$29.89/5g
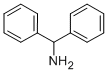
91-00-9
413 suppliers
$12.07/Price $/1gm:

3550-21-8
57 suppliers
$34.50/1g
Yield: 82%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in dichloromethane at 20; for 2 h;Cooling with ice;
Steps:
General procedure: To a stirred solution of thiophosgene (15.18 g, 132 mmol) in dried CH2Cl2 (150 mL) cooled in anice-water bath was added dropwise a solution prepared by dissolving 2c-2k, 2m-2n or 2ha-2hi (120mmol) and DIPEA (46.53 g, 360 mmol) in dried CH2Cl2 (150 mL). The resulting mixture was stirredfor 1 h in an ice-water bath and for another 1 h at room temperature. The reaction mixture was thenpoured into ice-water (300 mL) while stirring. The organic phase was separated, and the aqueousphase was back-extracted with CH2Cl2 (200 mL × 2). The combined organic phases were washedsuccessively with 5% hydrochloric acid (100 mL × 2; for 3f and 3n, the washing with 5% hydrochloricacid is omitted) and saturated brine (300 mL), dried (Na2SO4) and evaporated on a rotary evaporatorto give a residue, which was purified by column chromatography to afford 3c-3k, 3m-3n or3ha-3hi.
References:
Cai, Wenqing;Wu, Jingwei;Liu, Wei;Xie, Yafei;Liu, Yuqiang;Zhang, Shuo;Xu, Weiren;Tang, Lida;Wang, Jianwu;Zhao, Guilong [Molecules,2018,vol. 23,# 2,art. no. 252]
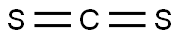
75-15-0
233 suppliers
$40.00/100 g

91-00-9
413 suppliers
$12.07/Price $/1gm:

3550-21-8
57 suppliers
$34.50/1g
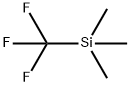
81290-20-2
398 suppliers
$13.00/1g

91-00-9
413 suppliers
$12.07/Price $/1gm:

3550-21-8
57 suppliers
$34.50/1g
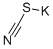
333-20-0
425 suppliers
$13.50/100G
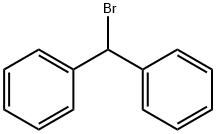
776-74-9
242 suppliers
$22.30/25g

3550-21-8
57 suppliers
$34.50/1g