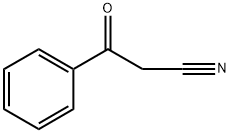
Benzoylacetonitrile synthesis
- Product Name:Benzoylacetonitrile
- CAS Number:614-16-4
- Molecular formula:C9H7NO
- Molecular Weight:145.16
Yield:614-16-4 90%
Reaction Conditions:
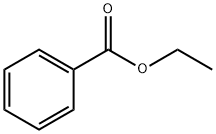
Stage #1:benzoic acid ethyl ester with potassium tert-butylate in tetrahydrofuran;water
Stage #2:acetonitrile in tetrahydrofuran;water at 20; for 0.5 h;Solvent;Time;
Steps:
4.5 Typical procedure for reaction of esters with cyanides to β-ketonitriles 9 under the optimized conditions
General procedure: Ethyl ester 1 (6.65 mmol, 1 equiv) was dissolved in THF (30 mL, technical grade involving 0.2% water) with stirring (about 230rpm) at ambient temperature for 5min. Potassium tert-butoxide (1.57 g, 14.0 mmol, 95%, 2 equiv) was added immediately to the above THF solution. After stirring enough the flask, the corresponding cyanide 8 (6.65mmol, 1equiv) was then added. The resulting mixture was stirred at ambient temperature. The reaction mixture was quenched by addition of water (50mL) and then stirred for 5min. After adding ethyl acetate (40 mL) and then HCl solution (1 mL, 12 M), the organic layer was separated and dried over anhydrous magnesium sulfate. The solvent was evaporated under reduced pressure, and the resulting residue was applied to the top of an open-bed silica gel column (for 9a-e, 9g-j: 3×15cm, n-hexane/ethyl acetate (3:1, v/v); for 9f: 3.5×8 cm, CH2Cl2). Fractions containing the product were combined and evaporated under reduced pressure to give the corresponding β-ketonitriles.
References:
Kim, Bo Ram;Lee, Hyung-Geun;Kang, Seung-Beom;Jung, Kwang-Ju;Sung, Gi Hyeon;Kim, Jeum-Jong;Lee, Sang-Gyeong;Yoon, Yong-Jin [Tetrahedron,2013,vol. 69,# 48,p. 10331 - 10336]
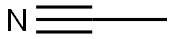
75-05-8
962 suppliers
$10.00/10g
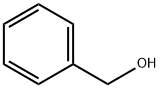
100-51-6
1368 suppliers
$5.00/100g

614-16-4
257 suppliers
$17.00/5g

17196-74-6
0 suppliers
inquiry

614-16-4
257 suppliers
$17.00/5g