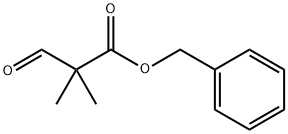
benzyl 2-formyl-2-methylpropanoate synthesis
- Product Name:benzyl 2-formyl-2-methylpropanoate
- CAS Number:97518-80-4
- Molecular formula:C12H14O3
- Molecular Weight:206.24
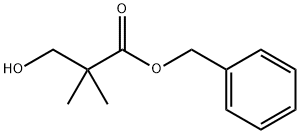
17701-61-0
40 suppliers
$24.00/250mg

97518-80-4
17 suppliers
inquiry
Yield:97518-80-4 82%
Reaction Conditions:
Stage #1: benzyl 3-hydroxy-2,2-dimethylpropanoatewith oxalyl dichloride;dimethyl sulfoxide in dichloromethane; for 1 h;Swern Oxidation;
Stage #2: with triethylamine in dichloromethane at 20;
Steps:
Benzyl 2,2-dimethyl-3-oxopropanoate
Oxalyl chloride (2.5 mL, 29.2 mmol) was added dropwise to a solution of DMSO (4.14 mL, 58.4 mmol) in CH2Cl2 (100 mL) at -78 °C. The reaction mixture was stirred for 10 min and a solution of alcohol 20 (5.0 g, 24.3 mmol) in CH2Cl2 (20 mL) was added. The solution was stirred for 1 h, NEt3 (13.5 mL, 97.3 mmol) added and the solution allowed to warm to rt. H2O (25 mL) was added and the mixture extracted with CH2Cl2 (3 50 mL). The combined organic extracts were washed with brine (30 mL), dried over Na2SO4 and concentrated under reduced pressure. Purification by column chromatography using 9:1 hexanes/EtOAc as eluent gave the title compound (4.13 g, 82%) as a yellow oil. Rf: 0.58 (80% hexanes/EtOAc); δH (400 MHz, CDCl3): 9.68 (1H, s, H-3), 7.39-7.31 (5H, m, Ph), 5.19 (2H, s, CH2Ph), 1.37 (6H, s, 2 CH3); δC (100 MHz, CDCl3): 198.9 (C=O, C-3), 172.5 (C=O, C-1), 135.3 (C, Ph), 128.6 (2 CH, Ph) 128.3 (CH, Ph), 127.9 (2 CH, Ph), 67.1 (CH2, CH2Ph), 45.9 (C, C-2), 19.6 (2 CH3). The spectroscopic data were in agreement with those reported in the literature.8
References:
Finch, Orla C.;Furkert, Daniel P.;Brimble, Margaret A. [Tetrahedron,2014,vol. 70,# 3,p. 590 - 596]

4835-90-9
215 suppliers
$13.00/25g

97518-80-4
17 suppliers
inquiry
100-39-0
424 suppliers
$10.00/10g

97518-80-4
17 suppliers
inquiry

14002-80-3
181 suppliers
$7.00/5g

97518-80-4
17 suppliers
inquiry