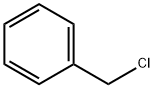
Benzyl chloride synthesis
- Product Name:Benzyl chloride
- CAS Number:100-44-7
- Molecular formula:C7H7Cl
- Molecular Weight:126.58
benzyl chloride is a lachrymator, and a powerful one at that. In the presence of water, it hydrolyzes to benzyl alcohol and hydrochloric acid, so it's clear why it burns your water saturated eye so bad.
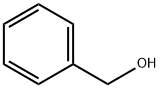
100-51-6
1368 suppliers
$5.00/100g
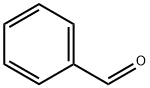
100-52-7
948 suppliers
$20.37/250g

100-44-7
627 suppliers
$13.50/250G
Yield:100-52-7 84.7% ,100-44-7 6.3%
Reaction Conditions:
Stage #1: benzyl alcoholwith oxalyl dichloride;dimethyl sulfoxide at 15;Flow reactor;Swern Oxidation;
Stage #2: with triethylamine at 15;Flow reactor;Swern Oxidation;Temperature;
Steps:
3.2. Experimental procedure
General procedure: The continuous ow microreactor system was assembled as shown in Figure 1. The Me2SO, activator, and benzyl alcohol were loaded into HPLC pumps with appropriate ow rates. After a stable period of 10 min, the effluent was collected in a separation funnel through which we could observe the color and layering by controlling the valve. Then effluent was finally collected into a continuously stirred ask containing an excess amount of triethylamine. The temperatures were kept constant by a water bath with constant circulation temperatures and a thermostat magnetism agitator. All of the reactions were monitored offline by GC-MS; the yields and selectivities were calculated by the method of area normalization. Upon completion of the reaction, the mixture of triethylamine and effluent was washed with 5% HCl and the organic layer was separated. Then the organic layer was washed 2 or 3 times until the mixture in the separation funnel reached neutral. In the process, the aqueous layers were always extracted twice with dichloromethane. At last, the organic layers were collected together and dried by anhydrous MgSO4 for 4 h. The final product, benzaldehyde, was obtained after filtration and reduced pressure distillation.
References:
Zhu, Lin;Xu, Xiaohui;Zheng, Fuping [Turkish Journal of Chemistry,2018,vol. 42,# 1,p. 75 - 85]

100-51-6
1368 suppliers
$5.00/100g

103-50-4
304 suppliers
$5.00/25g

100-44-7
627 suppliers
$13.50/250G

109-87-5
342 suppliers
$9.00/5g
71-43-2
671 suppliers
$11.19/25ML

100-44-7
627 suppliers
$13.50/250G

100-51-6
1368 suppliers
$5.00/100g

100-44-7
627 suppliers
$13.50/250G

100-52-7
948 suppliers
$20.37/250g

100-44-7
627 suppliers
$13.50/250G