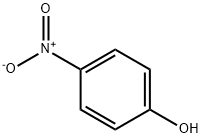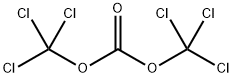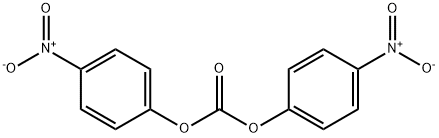
BIS(4-NITROPHENYL) CARBONATE synthesis
- Product Name:BIS(4-NITROPHENYL) CARBONATE
- CAS Number:5070-13-3
- Molecular formula:C13H8N2O7
- Molecular Weight:304.21

102-09-0
384 suppliers
$6.00/25g

5070-13-3
228 suppliers
$5.00/5g
Yield:5070-13-3 94%
Reaction Conditions:
with mixed-acid;sulfuric acid;nitric acid in cyclohexane;ethyl acetate;nitrobenzene
Steps:
1 Example 1
Example 1 Nitration of diphenyl carbonate in the presence of nitrobenzene. A 2000 ml reactor equipped with a mechanical stirrer, thermometer, and a jacketed addition funnel was charged with 107.11 g of diphenyl carbonate (0.5 mol) and 500 ml of nitrobenzene. The mixture was stirred until dissolution was complete. In the meantime, the mixed acid reagent was prepared by mixing 99.02 g of concentrated nitric acid and 125 ml of concentrated sulfuric acid in a beaker with cooling. The cooled mixed-acid reagent was then poured into the jacketed addition funnel and further cooled with ice. After the diphenyl carbonate had dissolved in the reaction vessel, the temperature was adjusted to 20° C. with an ice/water bath. The mixed-acid reagent was added at a rate which maintained the temperature of the reaction mixture at close to 20° C. The total addition took around 60 min. Following the addition, the reaction was stirred for an additional 60 min. The reaction mixture was then poured over 600 ml of ice/water. A 600 ml portion of ethyl acetate was added and the mixture transferred to a separatory funnel and shaken. The organic layer was removed and the aqueous layer was washed 3 times with 100 ml portions of ethyl acetate. The organic layers were combined and washed with 200 ml of saturated sodium bicarbonate and 200 ml of saturated brine. The organic layer was then dried over sodium sulfate. After the reaction mixture was dried, the ethyl acetate was removed by rotatory evaporation. The nitrobenzene solution of crude product was poured into 2000 ml of cyclohexane and the precipitated crude product (215.16 g) recovered by filtration. Gas chromatography showed the isomeric carbonates to be present in 95.6%, 4,4'-, 0.6%, 4,3'-, and 3.8%, 4,2'-dinitrodiphenyl carbonate, amounts. Recrystallization from a mixture of toluene and cyclohexane resulted in 142.7 g of di(4-nitrophenyl)carbonate or a 94% yield.
References:
Amoco Corporation US5037994, 1991, A
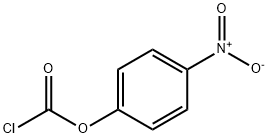
7693-46-1
417 suppliers
$18.00/5g

5070-13-3
228 suppliers
$5.00/5g

7693-46-1
417 suppliers
$18.00/5g
602-09-5
330 suppliers
$6.00/5g

5070-13-3
228 suppliers
$5.00/5g
138537-48-1
0 suppliers
inquiry