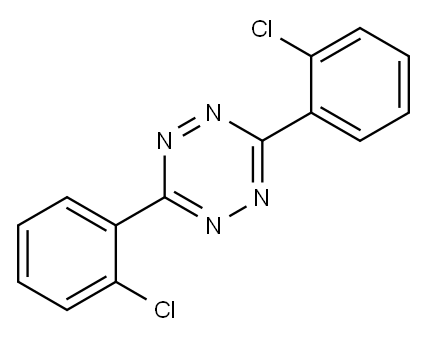
Clofentezine synthesis
- Product Name:Clofentezine
- CAS Number:74115-24-5
- Molecular formula:C14H8Cl2N4
- Molecular Weight:303.15

6830-78-0
47 suppliers
$50.00/10mg

74115-24-5
157 suppliers
$35.00/25mg
Yield:74115-24-5 35%
Reaction Conditions:
with N-chloro-succinimide;palladium diacetate;acetic acid at 120; for 17 h;Inert atmosphere;Schlenk technique;
Steps:
1.C 3,6-bis(2-chlorophenyl)-1,2,4,5-tetrazine
The 3,6-diphenyl-1,2,4,5-tetrazine (58.0 mg, 0.25 mmol), NCS (166.9 mg, 1.25 mmol), and Pd(OAc)2 (5.6 mg, 0.025 mmol) were introduced in a Schienk tube, equipped with a magnetic stirring bar. Acetic acid (2 mL) was added,and the Schienk tube purged several times with argon. The Schlenk tube was placed in a pre-heated oil bath at 120°C and reactants were allowed to stir for 17 h. After cooling to room temperature, the reaction mixture was diluted with dichloromethane, and was washed three times with water + 3% of TEA. The combined organic layer was washed with water and dried over MgSO4. Thesolvent was removed in vacuo and the residue was analyzed by NMR to determine the conversion of the chlorinated product. Then, the crude product was purified by silica gel column chromatography (Dichloromethane -Heptane = 1:1) to afford (4b) (purple solid) in 35% (29.4 mg) yield.‘H NMR (300 MHz, CDCI3): (ppm) = 8.13-8.10 (m, 2H), 7.66-7.63 (m, 2H),7.60-7.50 (m, 4H); ‘3C NMR (75 MHz, CDCI3): (ppm) = 165.1, 134.0, 132.8,132.5, 131.7, 131.4, 127.75; HRMS + p ESI (m/z) [M+H] Calcd forC14H8C12N4: 303.019. Found: m/z = 303.019.
References:
UNIVERSITE DE BOURGOGNE;HIERSO, Jean-Cyrille;ROGER, Julien;DECREAU, Richard;TESTA, Christelle WO2017/93263, 2017, A1 Location in patent:Page/Page column 30

74115-15-4
6 suppliers
inquiry

74115-24-5
157 suppliers
$35.00/25mg

74115-15-4
6 suppliers
inquiry

74115-24-5
157 suppliers
$35.00/25mg
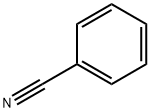
100-47-0
358 suppliers
$14.14/25gm:

74115-24-5
157 suppliers
$35.00/25mg

873-32-5
542 suppliers
$9.00/1g

74115-24-5
157 suppliers
$35.00/25mg