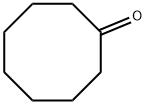
CYCLOOCTANONE synthesis
- Product Name:CYCLOOCTANONE
- CAS Number:502-49-8
- Molecular formula:C8H14O
- Molecular Weight:126.2
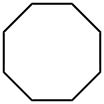
292-64-8
80 suppliers
$23.00/25mL

502-49-8
276 suppliers
$8.00/5g

696-71-9
78 suppliers
$45.60/25 mL
Yield:696-71-9 13% ,502-49-8 77%
Reaction Conditions:
with [Fe4III(μ-O)2(μ-acetate)6(2,2'-bipyridine)2(H2O)2](NO3-)(OH-);dihydrogen peroxide;acetic acid in water;acetonitrile at 32; for 3 h;Catalytic behavior;Overall yield = 89 %Spectr.;
Steps:
2.4. C-H activation analysis by the oxido-acetato-bridged tetraironcomplex (1)
General procedure: Conversion of cyclooctane: Cyclooctane (228 mg, 268 ml,2 mmol) was added to a solution of 1 (0.04 mmol) in MeCN:aceticacid (1.5:0.5 ml) in over all catalyst 1: substrate: oxidant0.04:200:500). After the addition of H2O2 (33% in H2O; 454 mL,5.0 mmol), the reaction mixture was heated at 32 °C for 3 h. Themixturewas then allowed to cool to room temperature. The organicphase was extracted with Et2O (3 x 1 ml), washed with brine anddried (MgSO4). After filtration, the solvents of the filtrate wereevaporated (rotary evaporator). The remaining mixture was separatedby column chromatography (silica gel; diethyl ether:pentane 1:20 as eluent) and the product was analyzed by GC-MSusing 1,2-dichlorobenzene as an internal standard.
References:
Dey, Dhananjay;Patra, Moumita;Al-Hunaiti, Afnan;Yadav, Hare Ram;Al-mherat, Afrah;Arar, Sharif;Maji, Milan;Choudhury, Angshuman Roy;Biswas, Bhaskar [Journal of Molecular Structure,2019,vol. 1180,p. 220 - 226]

696-71-9
78 suppliers
$45.60/25 mL

502-49-8
276 suppliers
$8.00/5g

292-64-8
80 suppliers
$23.00/25mL
201230-82-2
1 suppliers
inquiry

502-49-8
276 suppliers
$8.00/5g

4103-15-5
10 suppliers
$250.00/100mg

696-71-9
78 suppliers
$45.60/25 mL

502-49-8
276 suppliers
$8.00/5g
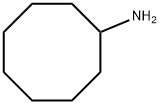
5452-37-9
98 suppliers
$5.00/250mg

292-64-8
80 suppliers
$23.00/25mL

502-49-8
276 suppliers
$8.00/5g