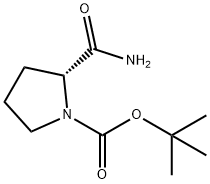
D-1-N-BOC-PROLINAMIDE synthesis
- Product Name:D-1-N-BOC-PROLINAMIDE
- CAS Number:70138-72-6
- Molecular formula:C10H18N2O3
- Molecular Weight:214.26
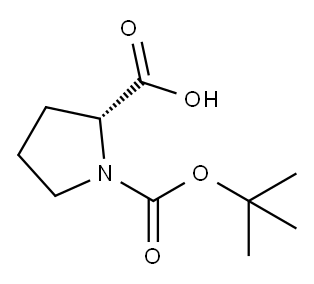
37784-17-1
410 suppliers
$6.00/5g

70138-72-6
122 suppliers
$9.00/1g
Yield:70138-72-6 54%
Reaction Conditions:
Stage #1:N-(tert-butoxycarbonyl)-D-proline with 4-methyl-morpholine;isobutyl chloroformate in tetrahydrofuran at -78; for 1 h;
Stage #2: with ammonia in tetrahydrofuran;water at -78 - 20; for 2 h;
Steps:
1
Example 1; (R)-2-Carbamoyl-pyrrolidine-1-carboxylic acid tert-butyl ester; N-Methylmorpholine (9.85 g, 97.5 mmol) and isobutyl chloroformate (13.3 g, 97.5 mmol) was added to (R)-pyrrolidine-1,2-dicarboxylic acid 1-tert-butyl ester (20.0 g, 92.9 mmol) in THF (200 mL) at -78° C. and stirred for 1 h. Ammonium hydroxide (58 mL) was added slowly as the reaction warmed up to RT and stirred for a further 2 h. The reaction mixture was partitioned between CH2Cl2 and water. The organic extracts were washed with 1 M HCl, dried over sodium sulphate, filtered and concentrated to afford the title product (10.8 g, 54%) as a colourless semisolid.1H NMR (300 MHz, CDCl3): δ (ppm) 5.91-6.13 (m, 1H), 4.17-4.30 (m, 2H), 3.37-3.48 (m, 2H), 2.10-2.18 (m, 2H), 1.84-1.96 (m, 2H), 1.45 (s, 9H).
References:
AstraZeneca AB;NPS PHARMACEUTICALS, INC. US2007/259926, 2007, A1 Location in patent:Page/Page column 9

73323-65-6
176 suppliers
$10.00/5g

70138-72-6
122 suppliers
$9.00/1g
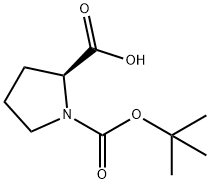
15761-39-4
591 suppliers
$5.00/10g

35150-07-3
247 suppliers
$5.00/1g

70138-72-6
122 suppliers
$9.00/1g
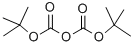
24424-99-5
815 suppliers
$13.50/25G

70138-72-6
122 suppliers
$9.00/1g
344-25-2
588 suppliers
$5.00/250mg

70138-72-6
122 suppliers
$9.00/1g