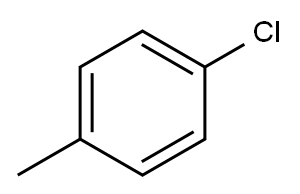
Di-p-tolylamine synthesis
- Product Name:Di-p-tolylamine
- CAS Number:620-93-9
- Molecular formula:C14H15N
- Molecular Weight:197.28
Yield:620-93-9 99%
Reaction Conditions:
with [Pd(N,N'-bis(2,6-bis(di-p-tolylmethyl)-4-methylphenyl)-imidazol-2-ylidene)(acetylacetonate)Cl];lithium hexamethyldisilazane in 1,4-dioxane at 110; for 3 h;Inert atmosphere;Sealed tube;Buchwald-Hartwig Coupling;
Steps:
2.3. Buchwald-Hartwig Cross-Coupling of Aryl Halides with Primaryor Secondary Amines: General Procedure
General procedure: A glassvial was charged with [Pd(IPr*me)(acac)Cl],neat amine (1.1 mmol), and the arylhalide (1 mmol) in dry 1,4-dioxane (1 mL) under an atmosphere of argonand sealed with a screw cap fitted with aseptum. LiHMDS (1.1 mmol) was subsequently injected at roomtemperature under argon, the reaction mixture was then refluxed at 110 for 3 h, After this time, dioxane was evaporated, the crude product wasdissolved in CH2Cl2. The solution was filtered on a padof silica covered with Celite, and thepad was eluted with CH2Cl2. After chromatography onsilica gel, the pure complex was obtained.
References:
Tian, Xiabing;Lin, Jing;Zou, Sheng;Lv, Junwei;Huang, Qingfei;Zhu, Jin;Huang, Shuping;Wang, Qiwei [Journal of Organometallic Chemistry,2018,vol. 861,p. 125 - 130] Location in patent:supporting information

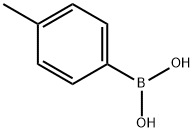
15738-23-5
26 suppliers
$128.00/500 mg
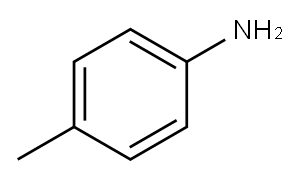
106-49-0
390 suppliers
$5.00/5 g

620-93-9
269 suppliers
$26.00/5g
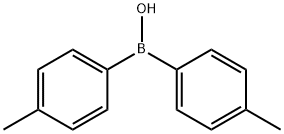
66117-64-4
30 suppliers
$45.00/100mg

106-49-0
390 suppliers
$5.00/5 g

620-93-9
269 suppliers
$26.00/5g