
Diisopropyl phosphite synthesis
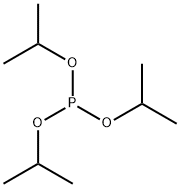
- Product Name:Diisopropyl phosphite
- CAS Number:1809-20-7
- Molecular formula:C6H15O3P
- Molecular Weight:166.16
3(CH3)2CHOH+PCl3→[(CH3)2CHO]2POH+2HCl↑+(CH3)2CHCl
At a ratio of 3:1 (mol), continuously feed 215kg/h of isopropanol and 150kg/h of phosphorus trichloride. When mixed, this results in a violent reaction that generates hydrogen chloride gas. This gas is quickly removed from the mixing pot using vacuum mercury. The crude ester produced is then heated in a falling film type spinner deacidification device and further deacidified. The resulting product is then cooled to below 40°C and obtained with a content of 92%-95% and a yield of 95%~98%.

67-63-0
1548 suppliers
$16.00/25ML

1809-20-7
174 suppliers
$8.00/10g
Yield:1809-20-7 92.1%
Reaction Conditions:
with phosphorus trichlorideCooling;
Steps:
2
(1) 138.8 g of phosphorus trichloride and 181.2 g of isopropyl alcohol were simultaneously charged into the continuous reactor (the molar ratio of phosphorus trichloride to isopropyl alcohol was 1: 3), and then cooled by vacuum O,O-diisopropyl phosphite 174.2 g, content: 92.1% chloroisopropane content 2.03%;(2) The O,O-diisopropyl phosphite obtained in the previous step was evacuated for 3 hours, the degree of vacuum was 0.02 MPa, the temperature was controlled at 30 to 40 ° C, O,O-diisopropyl phosphite The residual chlorinated isopropane was removed by bubbling, and the content of chloropropane was reduced to 0.22% after evacuation.
References:
Zhejiang jiahua Group co., LTD;HU, MIN DA;JI, BAO LIN;WU, ZHONG CN104356162, 2016, B Location in patent:Paragraph 0053-0055
116-17-6
174 suppliers
$14.00/5mL

1809-20-7
174 suppliers
$8.00/10g

116-17-6
174 suppliers
$14.00/5mL

123-11-5
834 suppliers
$10.00/10g

1809-20-7
174 suppliers
$8.00/10g

33472-11-6
5 suppliers
inquiry

2574-25-6
108 suppliers
$40.00/1g

1809-20-7
174 suppliers
$8.00/10g
