
Dodecanedioic acid synthesis
- Product Name:Dodecanedioic acid
- CAS Number:693-23-2
- Molecular formula:C12H22O4
- Molecular Weight:230.3
Yield:693-23-2 94.3 % ,1852-04-6 5.1 %
Reaction Conditions:
Stage #1: 1,2-dihydroxy-5,9-cyclododecenewith hydrogen;Ni/SiO2 at 160; under 4500.45 Torr;Autoclave;
Stage #2: with methanesulfonic acid;dihydrogen peroxide;acetic acid at 85;Reagent/catalyst;Temperature;
Steps:
2; 5-8 Example 2
Hydrogenation process: add 200 g of raw material 1,2-dihydroxy-5,9-cyclododecene (obtained after separation from 1,2-epoxy-5,9-cyclododecene after hydration reaction) into 1L In the autoclave, 3.0 g of 30% Ni-SiO2 hydrogenation catalyst was added, and the air in the autoclave was purged with nitrogen. Then feed the mixed gas of hydrogen and nitrogen into the reactor, the ratio of hydrogen and nitrogen in the mixed gas is 1:3. The reaction temperature is 160° C., the reaction pressure is 0.6 MPa, and the reaction time is 2 hours. After the reaction, the hydrogenation catalyst is removed by filtration or centrifugation, and the materials in the reactor are directly used in the oxidation process. According to gas chromatography analysis, the raw material conversion rate of the hydrogenation reaction is 99.9%, and the product ratio distribution in the hydrogenation reaction liquid system: 1-hydroxycyclododecanone accounts for 86.7%, 1-carbonylcyclododecanone accounts for 12.5%, 1,2-dihydroxycyclododecane accounts for 0.7%, and others account for less than 0.1%. Oxidation process: the hydrogenation process reaction solution from which the hydrogenation catalyst has been separated was placed in a 1L reactor, and 244g of acetic acid and 3g of methanesulfonic acid were added. Slowly add 207.8 g of 50% hydrogen peroxide dropwise at 85° C. for 1 h, and then continue stirring for 0.5 h. During the reaction, the product dodecanedioic acid was gradually precipitated. After the reaction is finished, the temperature of the reaction solution is cooled to room temperature, the crystalline solid is filtered out, and the conversion rate of the reaction and the yield of dodecanedioic acid are determined through esterification and derivatization. According to gas chromatography analysis, the raw material conversion rate of the oxidation reaction is 1006%, and the proportion distribution of solid products: dodecanedioic acid accounts for 94.3%, undecanedioic acid accounts for 5.1%, sebacic acid accounts for 0.5%, and other accounts for than less than 0.1%. The actual yield of dodecanedioic acid was 93.1%.
References:
CN115304471,2022,A Location in patent:Paragraph 0072-0084; 0092-0103
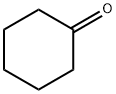
108-94-1
527 suppliers
$12.00/50g

693-23-2
407 suppliers
$5.00/25g

15199-41-4
28 suppliers
$90.00/100mg

693-23-2
407 suppliers
$5.00/25g

38279-34-4
5 suppliers
inquiry

4422-05-3
1 suppliers
inquiry

3956-80-7
2 suppliers
inquiry

693-23-2
407 suppliers
$5.00/25g

38279-34-4
5 suppliers
inquiry
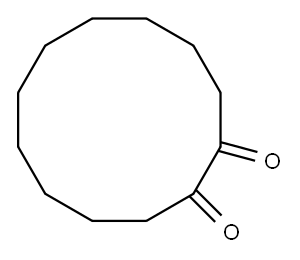
3008-41-1
5 suppliers
inquiry

