
Formamidine acetate synthesis
- Product Name:Formamidine acetate
- CAS Number:3473-63-0
- Molecular formula:C3H8N2O2
- Molecular Weight:104.11
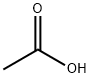
64-19-7
1530 suppliers
$10.00/25ML
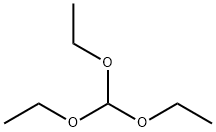
122-51-0
484 suppliers
$10.00/5ml

3473-63-0
479 suppliers
$5.00/25g
Yield:-
Reaction Conditions:
with ammoniaReflux;
Steps:
1; 2 Example 1
540 g (3.64 mol) of orthoformic acid triethyl ester and 200 g (3.33 mol) of glacial acetic acid were added to a 1,000 mL four-necked round bottom flask (reaction vessel) to which a stirrer, a reflux condenser, a gas inlet tube and a thermometer were connected, And introduction of an average of 10 g of anhydrous ammonia gas per hour was started while stirring under a nitrogen stream. At the start of introduction, the reaction vessel was immersed in a water bath at normal temperature. Introduction of anhydrous ammonia gas started from the state where the reaction solution temperature was normal, and the temperature of the reaction solution was allowed to rise naturally. When 50 g of anhydrous ammonia gas was introduced, the reaction liquid was heated by heating the water bath, and then the introduction of anhydrous ammonia gas was continued while maintaining the reflux state. A total of 118 g (6.93 mol) of anhydrous ammonia gas was introduced until completion of introduction. At this time, the reaction liquid was in a reflux state at the reflux temperature of ethanol and the reaction was completed. Subsequently, the reaction solution was cooled to 0 ° C. to sufficiently precipitate formamidine acetate salt, and solid-liquid separation was carried out by a conventional method to obtain wet crystals of formamidine acetate. The obtained formamidine vinegarThe acid salt wet crystals were dried under reduced pressure in a conventional manner to obtain 332 g (3.19 mol) of formamidine acetate. The yield based on acetic acid was 96%, and according to the purity analysis by the internal standard method using a high performance liquid chromatograph, the purity of formamidine acetate salt obtained was 99.7%. Further, when the filtrate was evaporated to dryness, 3% formamidine acetate was confirmed as a yield based on acetic acid. Therefore, the total yield (reaction yield) of formamidine acetate was 99% based on acetic acid.
References:
JP5722560,2015,B2 Location in patent:Sheet 0056; 0057
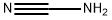
420-04-2
339 suppliers
$15.00/25g

64-19-7
1530 suppliers
$10.00/25ML

3473-63-0
479 suppliers
$5.00/25g

122-51-0
484 suppliers
$10.00/5ml

3473-63-0
479 suppliers
$5.00/25g